The Earth's
Atmosphere
The present atmosphere of the Earth is probably
not its original atmosphere. Our
current atmosphere is what chemists would call an oxidizing
atmosphere, while the original atmosphere was what chemists would call a
reducing atmosphere. In particular, it probably did not contain
oxygen.
Composition of the Atmosphere
The original atmosphere may have been similar to
the composition of the solar nebula and close to the present composition of the
Gas Giant planets, though this depends on the details of how the planets
condensed from the solar nebula.
That atmosphere was lost to space, and replaced by
compounds outgassed from the crust or (in some more recent theories)
much of the atmosphere may have come instead from the impacts of
comets and other planetesimals rich in volatile materials.
The oxygen so characteristic of our
atmosphere was almost all produced by plants
(cyanobacteria or, more colloquially,
blue-green algae). Thus, the
present composition of the atmosphere is 79% nitrogen, 20% oxygen, and 1% other
gases.
Layers of the Atmosphere
The atmosphere of the Earth may be divided into several distinct layers, as the
following figure indicates.
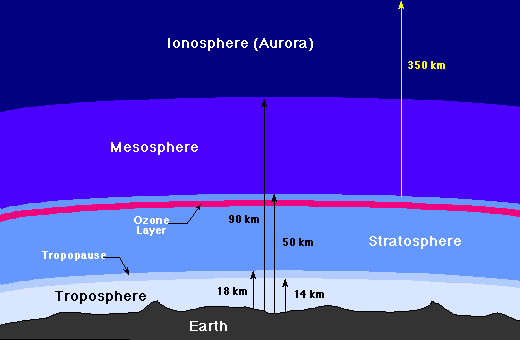 |
|
Layers of the Earth's atmosphere
|
The Troposphere
The troposphere is where all weather takes place; it is the
region of rising and falling packets of air.
The air pressure
at the top of the troposphere is only 10% of that at sea level (0.1
atmospheres). There is a thin buffer zone between the troposphere and the next
layer called the tropopause.
The Stratosphere and Ozone Layer
Above the troposphere is the
stratosphere, where air flow is mostly horizontal.
The thin
ozone layer
in the upper stratosphere has a high
concentration of ozone, a particularly reactive form of oxygen. This layer is
primarily responsible for absorbing the ultraviolet radiation from the Sun.
The formation of this layer is a delicate matter, since only when oxygen is
produced in the atmosphere can an ozone layer form and prevent an intense flux
of ultraviolet radiation from reaching the surface, where it is quite hazardous
to the evolution of life. There is considerable recent concern that
manmade flourocarbon compounds may be depleting the ozone layer, with dire
future consequences for life on the Earth.
The Mesosphere and Ionosphere
Above the stratosphere is the mesosphere and above that is the
ionosphere (or thermosphere),
where many atoms are ionized (have gained or
lost electrons so they have a net electrical
charge). The ionosphere is very thin, but it is where aurora take place, and
is also responsible for absorbing the most energetic photons from the Sun, and
for reflecting radio waves, thereby
making long-distance radio
communication possible.
The structure of the ionosphere is strongly influenced by the charged particle
wind from the Sun
solar wind, which is in turn governed by the level of Solar activity. One
measure of the structure of the ionosphere is the free electron density, which
is an indicator of the degree of ionization.

