Molecules

|
Atoms and Molecules |
The basic building blocks of the "normal" matter that we see in the Universe are atoms, and combinations of atoms that we call molecules. We first consider atoms and then molecules. However, we shall see that although "normal matter" is composed of atoms and molecules, most of the matter in the Universe is not in the form of atoms or molecules, but rather in the form of a plasma. We discuss plasmas in the next section.
| Constituent | Symbol | Charge | Mass |
| Electrons | e- | -1 | 9.1 x 10-28 g |
| Protons | p+ | +1 | 1836 x electron mass |
| Neutrons | n | 0 | Approximately that of p+ |
The number of protons (or the number of electrons) is called the atomic number for the atom. The total number of protons plus neutrons is called the atomic mass number for the atom. Atoms are electrically neutral because the number of negatively-charged electrons is exactly equal to the number of positively-charged protons. The number of neutrons is approximately equal to the number of protons for stable light nuclei, and is about 1-2 times the number of protons for the heavier stable nuclei.
Because atoms are neutral, most of the mass of atoms resides in the neutrons and protons which occupy the dense central region called the nucleus (see the Bohr atom below).
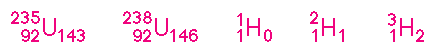
In this notation the element is represented by its chemical symbol, the atomic number is denoted by a lower left subscript, the number of neutrons is denoted by a lower right subscript, and the atomic mass number is denoted by an upper left superscript (some of these superscripts and subscripts may be omitted, depending on the context).
Thus, the above symbols denote, respectively, the mass-235 and mass-238 isotopes of uranium (symbol U), and the mass-1,-2,and -3 isotopes of hydrogen (symbol H). The mass-2 isotope of hydrogen is also called deuterium and the mass-3 isotope is also called tritium.