 |
| Curve of binding energy. |
| The Curve of Binding Energy |
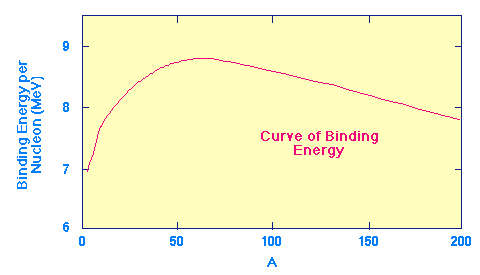
The key to energy production in stars lies in what nuclear physicists call the curve of binding energy, which is illustrated in the following figure.
 |
| Curve of binding energy. |
This plot shows the amount of binding energy per nucleon (A nucleon is either a neutron or a proton. The nucleon number is the sum of the number of neutrons plus protons in a nucleus; thus, it is equal to the atomic mass number) as a function of the atomic mass number A. The energy units are MeV, which stands for "million electron-volts", a standard unit of energy in nuclear physics.
This curve indicates how stable atomic nuclei are; the higher the curve the more stable the nucleus. Notice the characteristic shape, with a peak near A=60. These nuclei (which are near iron in the periodic table and are called the iron peak nuclei) are the most stable in the Universe. The shape of this curve suggests two possibilites for converting significant amounts of mass into energy. Note that this is the "upside down" version of the similar graph in text. There the vertical scale increases downward, here it increases upward.
In both fission and fusion reactions the total masses after the reaction are less than those before. The "missing mass" appears as energy, with the amount given by the famous Einstein equation.