Model
 |
The Bohr Model |
The most important properties of atomic and molecular structure may be exemplified using a simplified picture of an atom that is called the Bohr Model. This model was proposed by Niels Bohr in 1915; it is not completely correct, but it has many features that are approximately correct and it is sufficient for much of our discussion. The correct theory of the atom is called quantum mechanics; the Bohr Model is an approximation to quantum mechanics that has the virtue of being much simpler. (Here is a more realistic discussion of what atomic orbitals look like in quantum mechanics.)
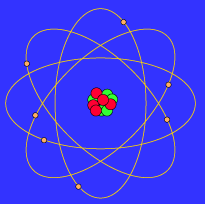 |
| The Bohr atom |
This similarity between a planetary model and the Bohr Model of the atom ultimately arises because the attractive gravitational force in a solar system and the attractive Coulomb (electrical) force between the positively charged nucleus and the negatively charged electrons in an atom are mathematically of the same form. (The form is the same, but the intrinsic strength of the Coulomb interaction is much larger than that of the gravitational interaction; in addition, there are positive and negative electrical charges so the Coulomb interaction can be either attractive or repulsive, but gravitation is always attractive in our present Universe.)
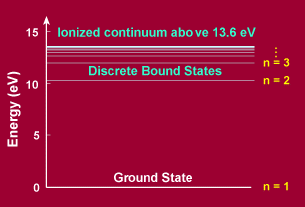 |
| Quantized energy levels in hydrogen |
The adjacent figure shows such quantized energy levels for the hydrogen atom. These levels are labeled by an integer n that is called a quantum number. The lowest energy state is generally termed the ground state. The states with successively more energy than the ground state are called the first excited state, the second excited state, and so on. Beyond an energy called the ionization potential the single electron of the hydrogen atom is no longer bound to the atom. Then the energy levels form a continuum. In the case of hydrogen, this continuum starts at 13.6 eV above the ground state ("eV" stands for "electron-Volt", a common unit of energy in atomic physics).
Although this behavior may seem strange to our minds that are trained from birth by watching phenomena in the macroscopic world, this is the way things behave in the strange world of the quantum that holds sway at the atomic level.
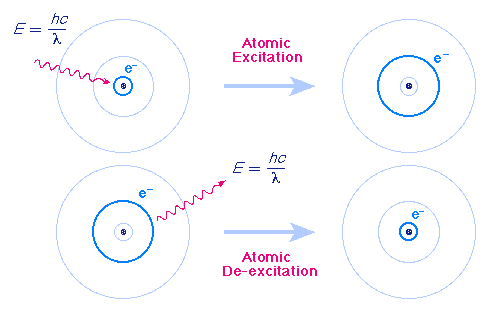 |
| Excitation by absorption of light and de-excitation by emission of light |
In each case the wavelength of the emitted or absorbed light is exactly such that the photon carries the energy difference between the two orbits. This energy may be calculated by dividing the product of the Planck constant and the speed of light hc by the wavelength of the light). Thus, an atom can absorb or emit only certain discrete wavelengths (or equivalently, frequencies or energies).