The modern atomic theory of gases tells us that a
gas is made up of many small particles (atoms or molecules) such that the
particles are spaced far apart from each other on the length scale of the
size of the particles. We can imagine the following simple model for the
behavior of these particles, which is not too far from the truth. Each
particle of the gas is undergoing a random motion as follows: it moves
with a constant speed ![]() in a given direction, until it collides with another particle, after which
it bounces off with speed
in a given direction, until it collides with another particle, after which
it bounces off with speed ![]() in a new random direction. How fast are these particles in a gas moving?
How big is the speed
in a new random direction. How fast are these particles in a gas moving?
How big is the speed ![]() ?
?
To answer this question, we can view the motion of
the particle in the above model, as if it is taking a random walk. The
distance L of the steps in the walk is just the distance the particle travels
inbetween two sucessive collisions. The time ![]() is just the time inbetween two sucessive collisions. If the speed inbetween
collisions is
is just the time inbetween two sucessive collisions. If the speed inbetween
collisions is ![]() ,
then L and
,
then L and ![]() are related by
are related by ![]() ,
or equivalently
,
or equivalently ![]() .
The diffusion constant for the particles of the gas is therefore:
.
The diffusion constant for the particles of the gas is therefore:
![]()
If me measure D in an experiment, and if we know
L, then we can use the above result to calculate the speed ![]() of the atomic or molecular particles of the gas.
of the atomic or molecular particles of the gas.
Instead of trying to measure the diffusion constant
of a molecule in a gas, we do instead the following simpler experiment
in lecture. A simple example of diffusion is to place a drop of dark blue
ink in a bowl of water. Initially the ink is all concentrated near one
position. As time increases, one sees the spot spread out and get fainter,
until after a sufficiently long time, the entire bowl of water is a uniform
light pale blue. The decrease in the intensity of the color of the spot,
from an initial dark blue to a final very light pale blue, is a result
of the decreasing concentration of ink as it diffuses into the water. If
one measured the spreading of the spot, one would find that it grows in
time like ![]() .
The particles of ink are taking a random walk as they collide with the
water molecules.
.
The particles of ink are taking a random walk as they collide with the
water molecules.
Note that the fact that the ink does indeed diffuse into the water is itself suggestive evidence that the ink and the water are both indeed made of many tiny particles which are colliding and so undergoing random walks. This is far from obvious; when one first looks at water or ink, they look like smooth continuous substances rather being made up of discrete small particles. Without any microscope to directly see the particles, the observation of diffusion gives suggestive evidence for the atomistic nature of matter!
In our experiment in class, we found that the ink spread out approximately 1/4 inch in 2 minutes. This gives a diffusion constant of
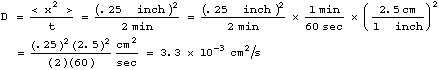
where we have used the facts that there are 60 seconds
in one minute, and 2.5 centimeters in one inch, to convert the units of
D from square inches per minute to square centimeters per second (![]() ).
The later units are those most commonly used in physics.
).
The later units are those most commonly used in physics.
We now want to use our measured value of D to determine the speed v of the ink particles, as the travel inbetween collisions with the water molecules. Since D=Lvo, we still need to determine L, the distance traveled between collisions. We will make the reasonable assumption that the distance between collisions is just the average distance between water molecules.
If you remember your elementary chemistry or physics,
you should recall that the length of typical atomic distances is the "Angstrom",
denoted by the symbol Å, and having the value, Å=![]() .
So we could guess that the distance between water molecules L should be
a few Å. If you remember even more of your elementary chemistry,
we can get a better estimate for L by the argument below:
.
So we could guess that the distance between water molecules L should be
a few Å. If you remember even more of your elementary chemistry,
we can get a better estimate for L by the argument below:
If one has a volume V filled with N molecules of
water, then the density of molecules per unit volume is ![]() (
(![]() is Greek letter
"rho"). The amount of volume per molecule is then
is Greek letter
"rho"). The amount of volume per molecule is then ![]() .
If L is the separation between water molecules, then this volume per molecule
is just
.
If L is the separation between water molecules, then this volume per molecule
is just ![]() .
So combining these results we conclude that
.
So combining these results we conclude that ![]() ,
or
,
or ![]() . So
if we know the density of water molecules
. So
if we know the density of water molecules ![]() ,
we can find L.
,
we can find L.
Now from elementary chemisty (we will review this
later, if you don't remember it now) we know that one "gram molecular weight"
of water, ![]() ,
weighs 18 grams. One gram molecular weight of something contains one Avogradro's
number,
,
weighs 18 grams. One gram molecular weight of something contains one Avogradro's
number, ![]() of molecules of
the stuff. So there are
of molecules of
the stuff. So there are ![]() molecules of water in 18 grams of water, or
molecules of water in 18 grams of water, or ![]() molecules in one gram of water. Now 1 gram of water at room temperature
occupies
molecules in one gram of water. Now 1 gram of water at room temperature
occupies ![]() of volume. So
of volume. So ![]() of water contains
of water contains ![]() molecules,
giving a density of
molecules,
giving a density of ![]() .
The distance between collisions is therefore
.
The distance between collisions is therefore
![]()
Using our measured value of ![]() ,
and
,
and ![]() , we now use
, we now use ![]() ,
or
,
or ![]() , to
find the speed of the ink particles inbetween collisions,
, to
find the speed of the ink particles inbetween collisions,
![]()
To get a better idea of how fast this is, lets convert back to more familiar units:
![]()
![]()
so
![]()
Pretty fast!!
We can also now compute the collision time ![]()
![]()
So the collision time is really short!
One of our goals towards the end of the course will
be to understand why this speed ![]() is so fast.
is so fast.
Some additional comments:
You could wonder how our simple random walk, where
in each step we move only a fixed amount either forward or backward, could
be a good model of random molecular collisions. Clearly the distance between
collisions is not always exactly L, but only on average L. In each particular
collisions the actual distance gone depends on the velocity of the molecule,
and the velocity and positions of the nearby molecules - certainly a distributions
of distances is possible. Suppose we describe this with a probability distribution
P(x), the probability to go distance x between two successive collisions
(x has positive sign for forward, negative sign for backward). Clearly
the mean distance of the probability distribution P(x) is zero - on average
the molecule goes just as much to the right as to the left. But P(x) has
some non zero root mean square average (the standard deviation), which
we will call L. Now the distance gone in N steps is just the result of
adding the outcomes of the probability distribution P(x), N times. We know
that the standard deviation of this sum of N elementary displacements is
then just ![]() L.
This is all we need to get diffusion - the details of the elementary displacement
probability distribution P(x) don't really matter - only the root mean
square average distance in one step L is important.
L.
This is all we need to get diffusion - the details of the elementary displacement
probability distribution P(x) don't really matter - only the root mean
square average distance in one step L is important.
For our simple random walk in one dimension (a walk
along a line) we saw that the probability to remain at the origin decreased
with the number of steps as ![]() .
This was a consequence of the width growing as
.
This was a consequence of the width growing as ![]() .
For a random walk in two dimensions, the width of the distribution grows
like
.
For a random walk in two dimensions, the width of the distribution grows
like ![]() in
each of the two orthogonal directions. Thus the area over which a sizable
probability gets spread out goes like
in
each of the two orthogonal directions. Thus the area over which a sizable
probability gets spread out goes like ![]() .
If the total amount of probability must always sum to unity, this suggests
that the height of the peak of the probability distribution (which is the
probability to remain at the origin) must decrease as 1/N. For a random
walk in three dimensions, the probability gets spread out over a volume
that grows like
.
If the total amount of probability must always sum to unity, this suggests
that the height of the peak of the probability distribution (which is the
probability to remain at the origin) must decrease as 1/N. For a random
walk in three dimensions, the probability gets spread out over a volume
that grows like ![]() .
So the height of the peak must decrease as
.
So the height of the peak must decrease as ![]() .
.
Since in diffusion, the number of steps N is proportional
to the time t, for a process in which diffusion is limited to one direction
only, the concentration at the origin decreases in time as ![]() ;
for a process in which diffusion takes place in two independent directions
the concentration at the origin decreases in time a
;
for a process in which diffusion takes place in two independent directions
the concentration at the origin decreases in time a ![]() ;
and for a process in which diffusion takes place in all three directions
in space, the concentration decreases in time as
;
and for a process in which diffusion takes place in all three directions
in space, the concentration decreases in time as ![]() .
.