Quantum effects are important in this size range and, based on my knowledge of theory, the many-body glue potential will not be appropriate to account for such effects or allow reliable structure calculations in this size regime.
As Christoph clarified, the glue pontential is an empirical model with parameters fitted to include the quantum effects of electronics. Many studies of small gold clusters have been done with glue potential, most of them are with the sizes smaller than ours. Ercolessi et al. [2] used the glue potential to investigate the structure of small gold particles with N=100-900 atoms. As they pointed out, The reliability of this potential is questionable when N<=200, where several atoms have a small coordination number (<8), while the potential is fitted on bulk and surface where coordination is larger. We studied the clusters with a few thousand atoms, which is far from the questionable size range, and should give reasonable results. Besides, with today's computers, it is still impossible to study such large clusters by ab initio methods, so work can only be done by empirical methods, such as the glue potential, EAM, etc.The use of bond orientational parameters on pages 3 and 4 classified according to symmetry is most likely not commonly understood. Hence these quantities should be defined in sufficient detail, such that non-specialists may understand the reasoning.
The limited size of PRL paper doesn't allow us to put the details of bond order parameters on. Readers would find the details in the original paper we refered. However, we may add some words to describe the general idea of bond order parameters.The division into surface and bulk layers on page 3 seems rather arbitrary. Considering that surface relaxations die off very fast with deeper layers, it seems to be more reasonable for such small clusters to limit the surface to two or at most three layers, and take the rest as bulk. How does this affect plots 2a and 2b?
We analyzed all layers and only the surface layer and the interior are shown in Fig. 2 because of the limited size of the paper. Other layers behave trivially between them. The plots are shown below.Surface layer
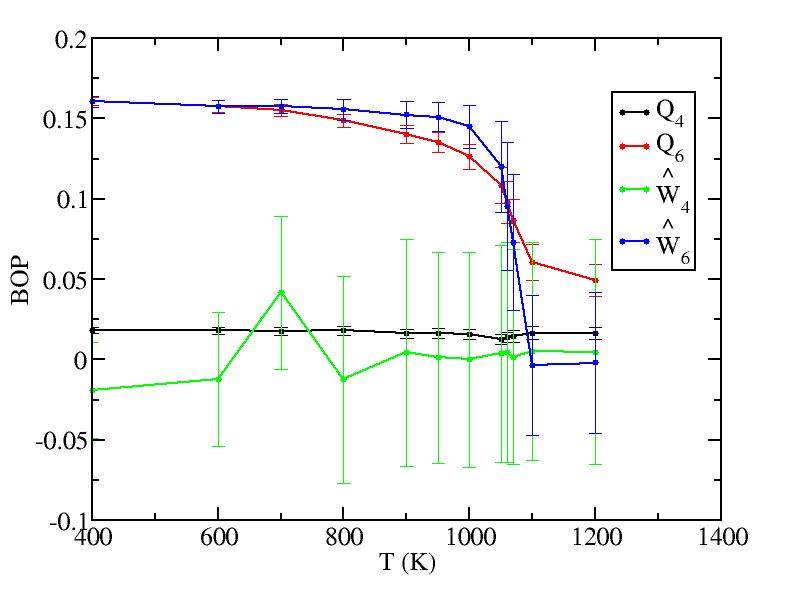
Sub layer 1
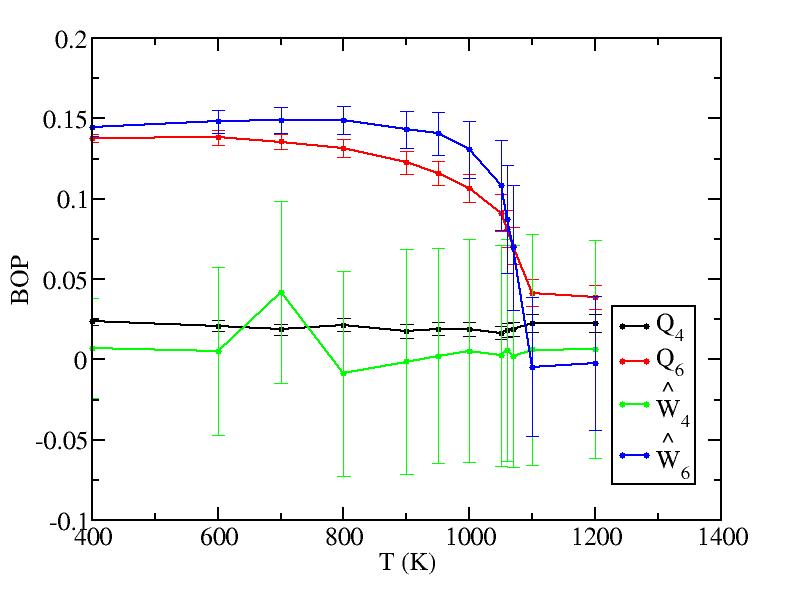
Sub layer 2
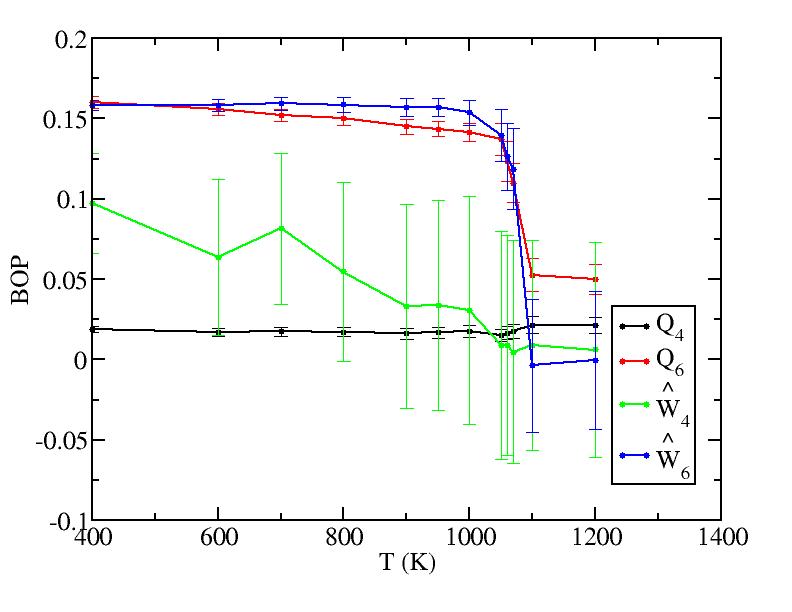
Sub layer 3
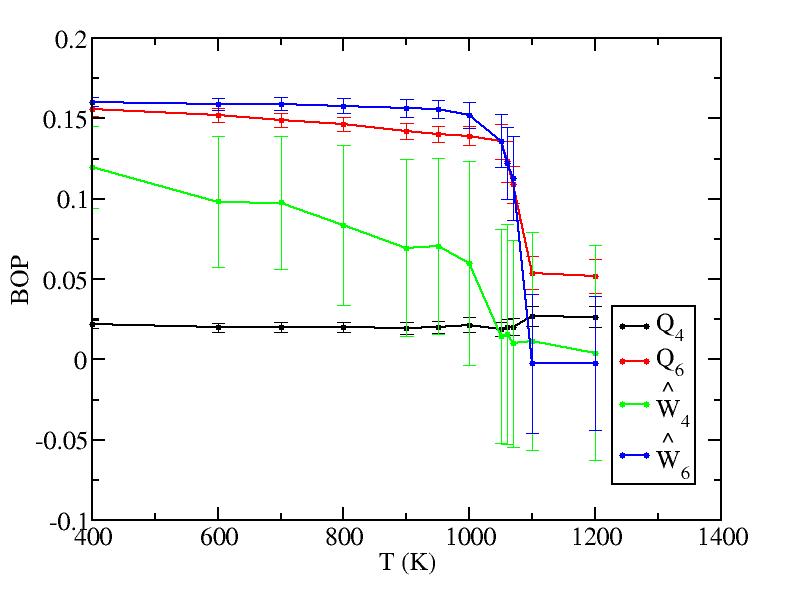
Sub layer 4
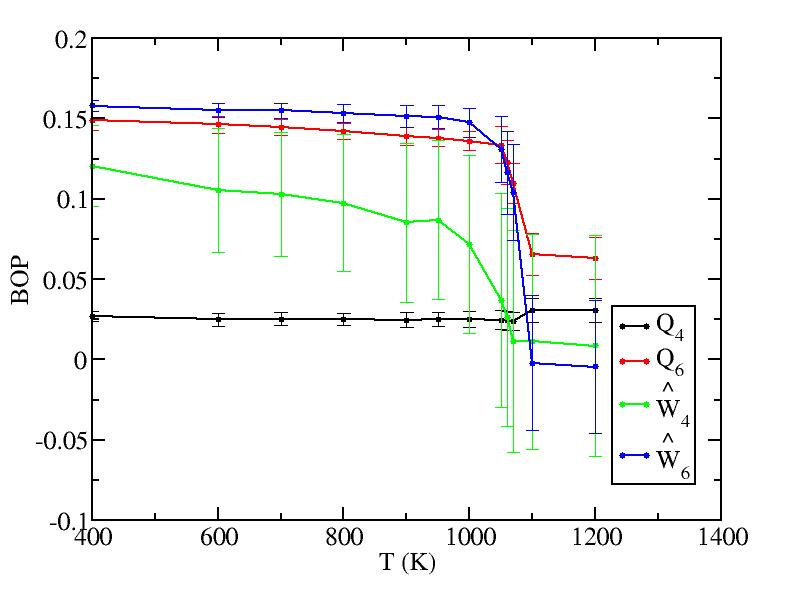
Bulk
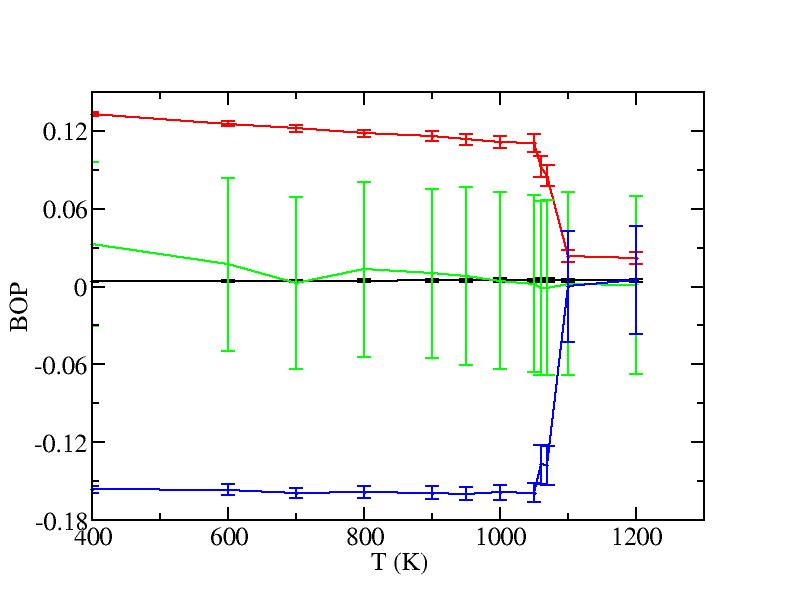
As referee B suggested, we also treat the outmost first two layers as surface and the rest as bulk to calculate their bond orientational parameters. The plots are shown below.
Surface (first two layers)
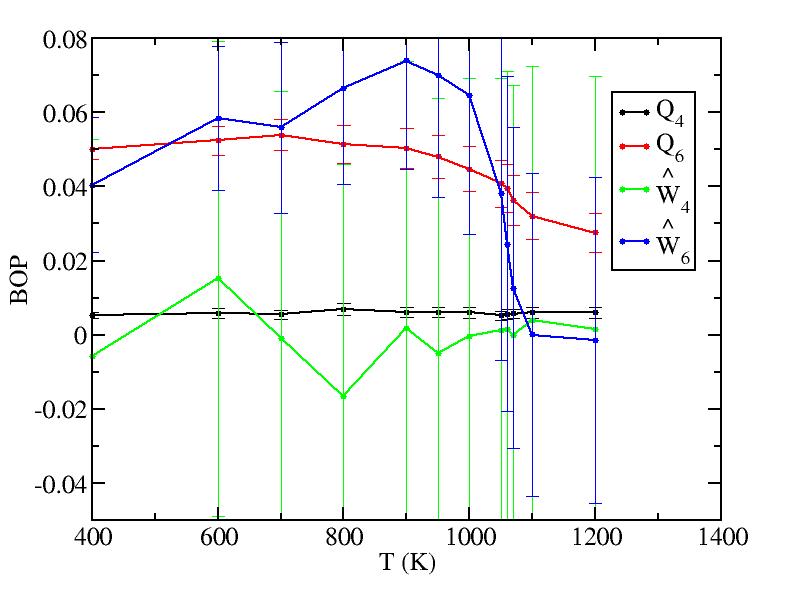
Bulk (all other interior atoms)
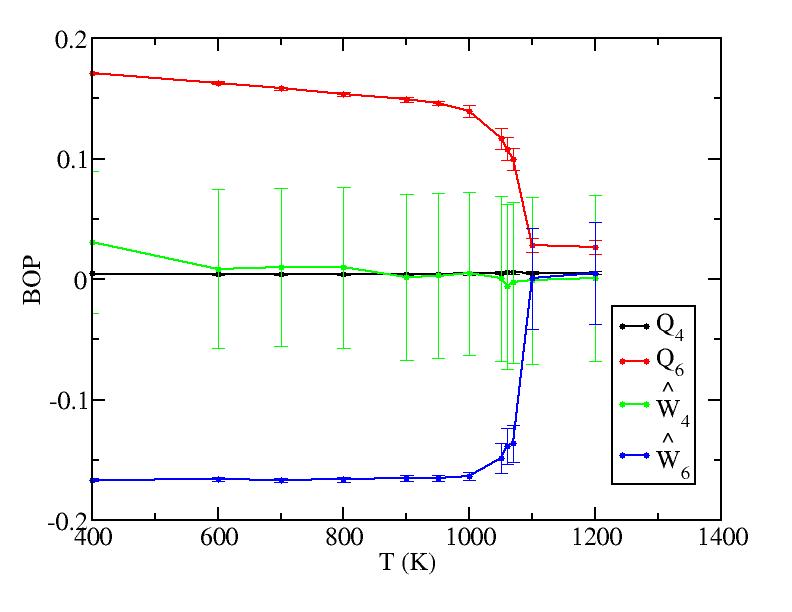
The bond order parameters for bulk just act as we expect from the mixing. Those for surface now become odd because now we include the bonds between two layers, whose vibrations severely interfere the values of the bond order parameters.
Fig. 2a, what is the meaning of a negative order parameter?
The negative values can be found in the original paper by Steinhardt et al. (ref. 29). (Should we explain when the negative values present?)From plot 3b it should be possible to extract the activation energy of surface self-diffusion. Does this yield a reasonable value compared to experiments?
The plot of ln(D) vs. 1/T is shown below.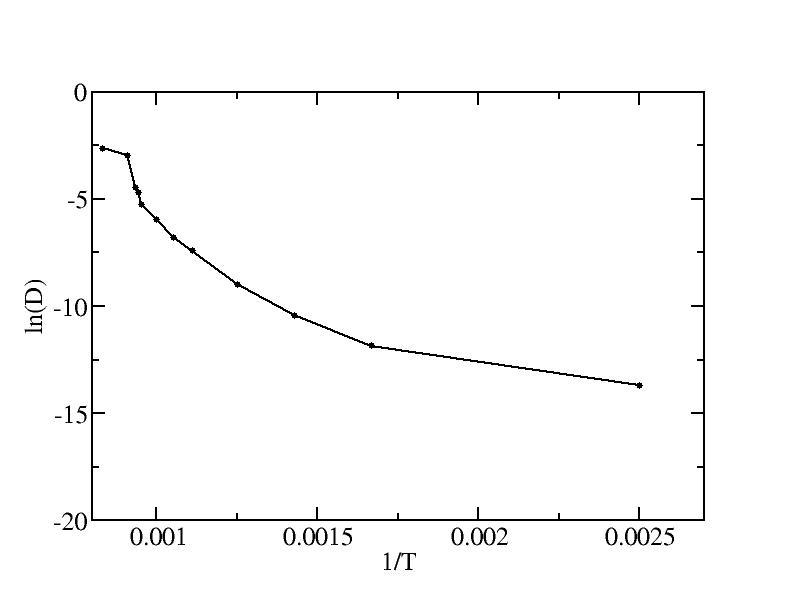
We fit the lines for 4 temperature ranges according to the emperical Arrhenius law D = D0 * exp( -EA/T ):
- 400K - 600K:
EA = 3.42*10-20 J = 0.21 eV
D0 = 5.45*10-4 Angstrom2 / ps - 700K - 950K:
EA = 1.15*10-19 J = 0.72 eV
D0 = 5.62 Angstrom2 / ps - 1050K - 1070K:
EA = 7.32*10-19 J = 4.58 eV
D0 = 4.36 * 1019 Angstrom2 / ps - 1100K - 1200K (liquid):
EA = 5.61*10-20 J = 0.35 eV
D0 = 2.14 Angstrom2 / ps
To our knowledge, no experimental activation energy data for Au {111} facets are currently available. We found two papers with the simulation results:
- Chushak and Bartell [4] reported their the activation energy for
their simulated liquid gold nanoclusters by EAM model (Page
11608, below Eq. 11):
EA = 23.88 kJ/mol = 3.97*10-20 J = 0.25 eV
D0 = 27.9*10-9 m2/s = 2.79 Angstrom2 / psConsidering the tendency of EAM models to systematically give lower energy values as pointed out by ref. [5] and many other resources, our result for the liquid gold cluster is in very good agreement with theirs.
- Boisvert et al. [5] did the first principles calculations for the bulk Au {111} surface at low temperature and the activation energy they found is (Table V): EA = 0.22 +/- 0.03 eV. Our result of EA = 0.21 eV for T<=600K is a perfect match with their first principles result.
Fig. 4, is the shape at 600 K the same as at lower temperature? What are the criteria for this shape to be an equilibrium rather than a kinetically limited form? Why is it not highly symmetric if all flats are {111} facets? Also, I do not recognize this cluster to be an icosahedron with 20 (almost) equal {111} facets (claim on page 2).
The shape at 600K is almost the same as at lower temperature except that the vertices is a little rounded. Those shapes are averaged over very long equilibrating time at each temperature, so it should be an equilibrium form, not a kientically limited one. Because the size of 2624 is not a magic number, the formed ih clusters has 20 {111} facets with different sizes. The clusters with magic numbers we studied are highly symmetric. The pictures in the first row of Fig. 4 are only a profile of cluster surfaces to show the rounding of vertices and edges. We carefully examined the numbers of vertices and edges and the structure of surfaces to ensure what we got are really icoshedra with 20 {111} facets (not necessary to be equal large). Below we show the same average shape of 600K colored with max local curvature from another perspective (The particles are not real surface atoms but the average atom positions in the solid angle cells).