January 21 2003
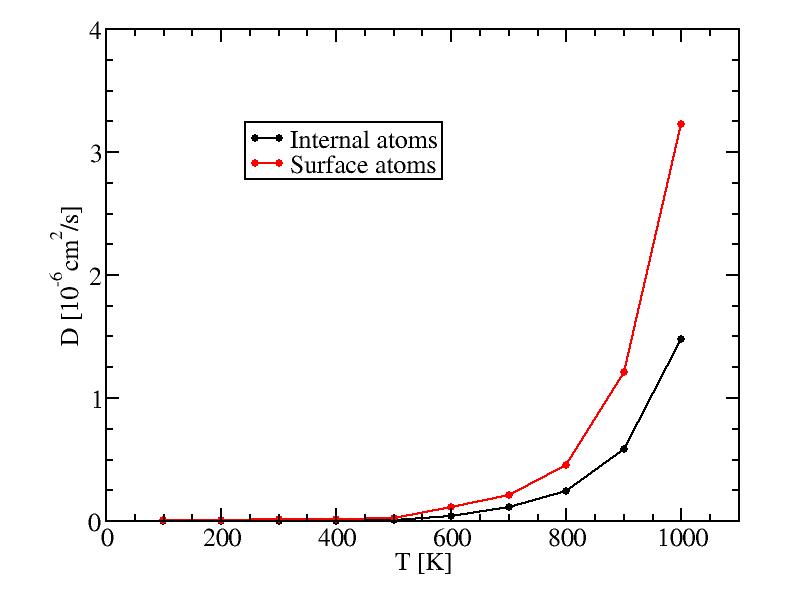
Diffusion coefficients for 2624 atoms (heating results for T>=1000K)
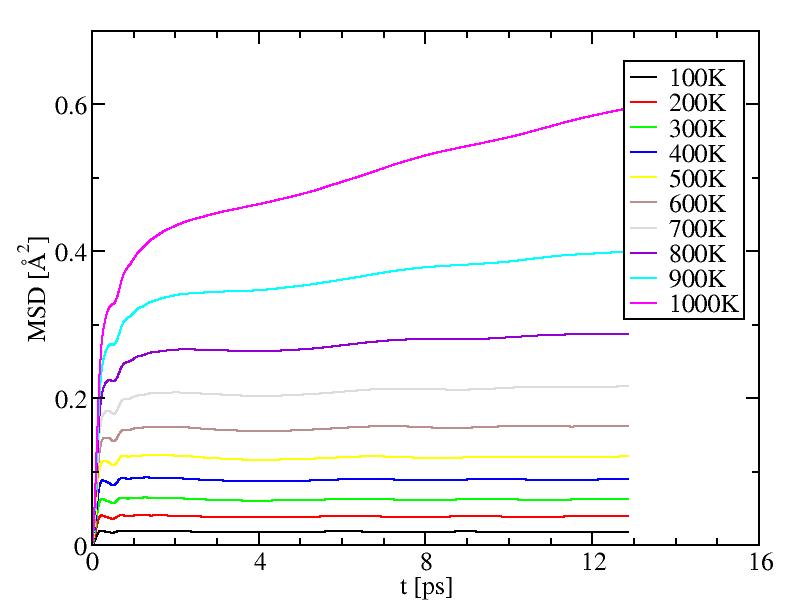
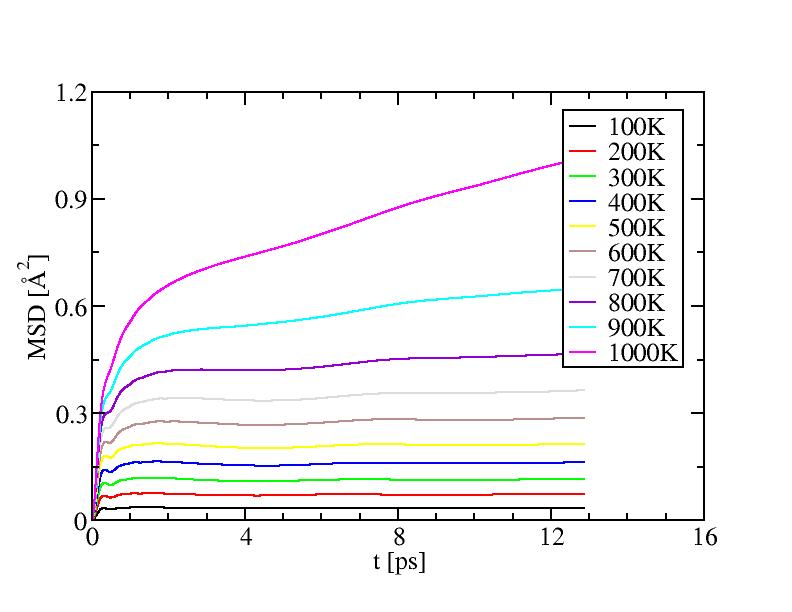
The results of diffusion coefficient and diffusion between layers both
suggest the surface melting of this icosahedral system starts at a temperature
between 500K and 600K. ( Can surface also be supercooled? If so, our cooling
procedure may lowered this temperature. ) Surface melting seems to be a
second-order transition.
Diffusion coefficients were collected by constant temperature MD simulations
from the end point of corresponding Andersen thermostat simulations.
Mean square displacements for internal atoms
Mean square displacements for surface atoms
Diffusion coefficients
Diffusion between layers
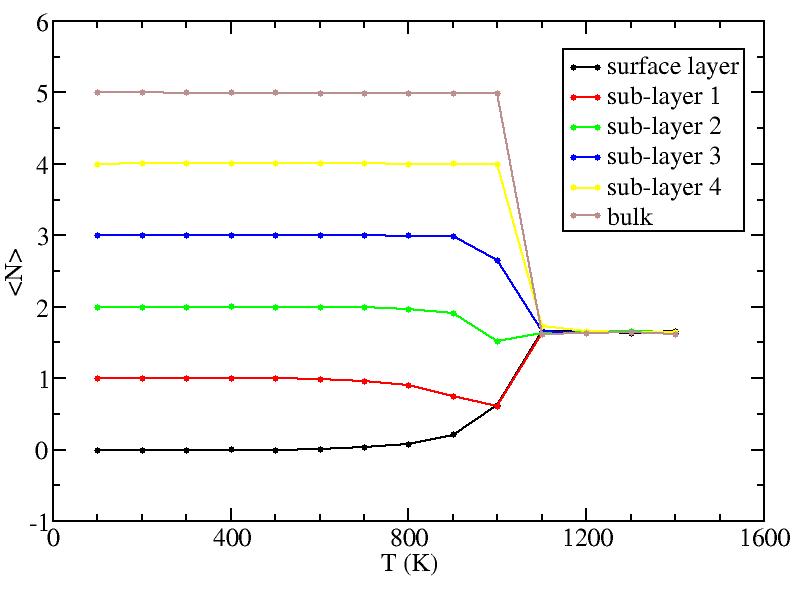
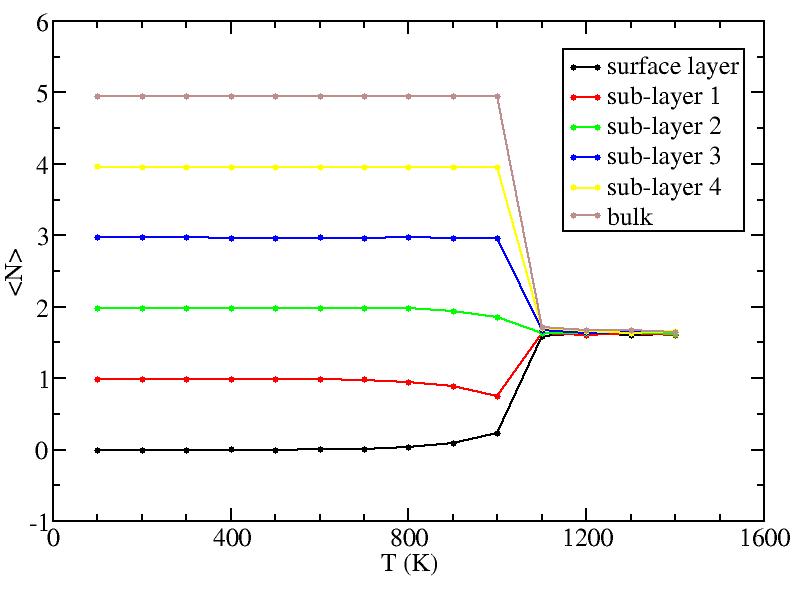
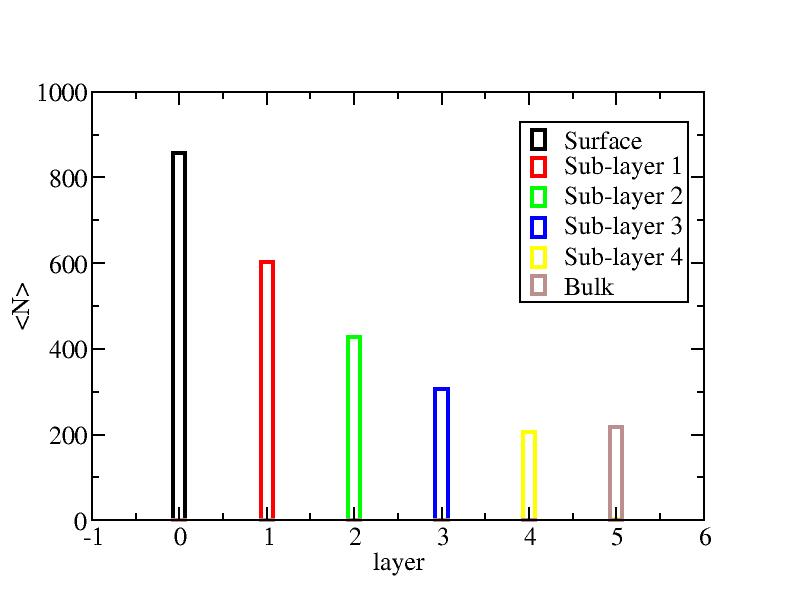
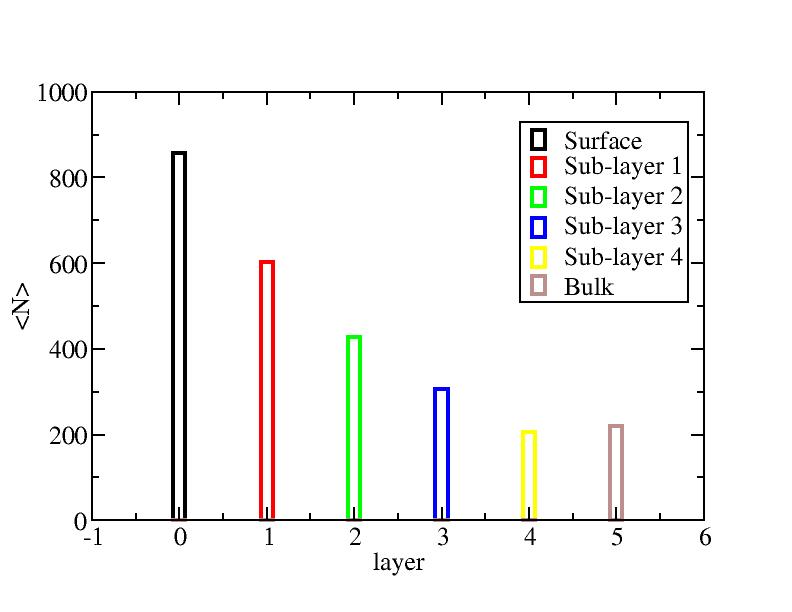
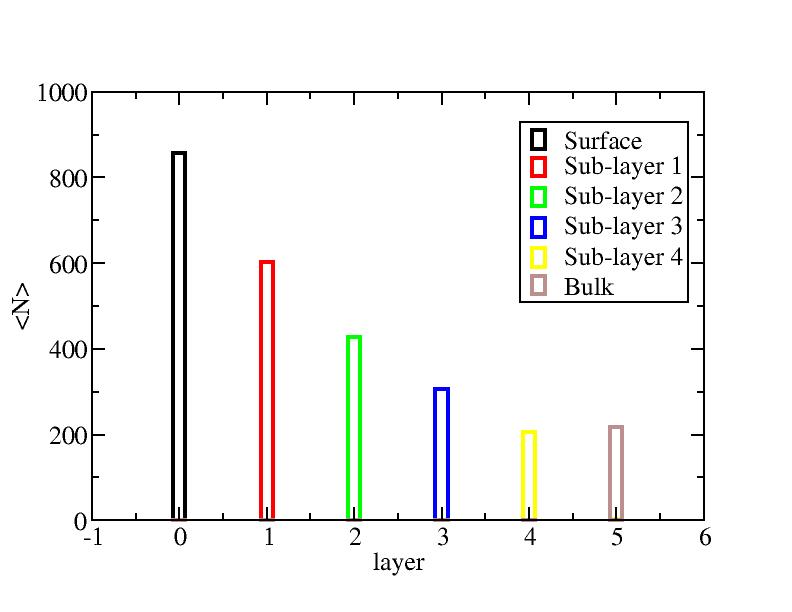
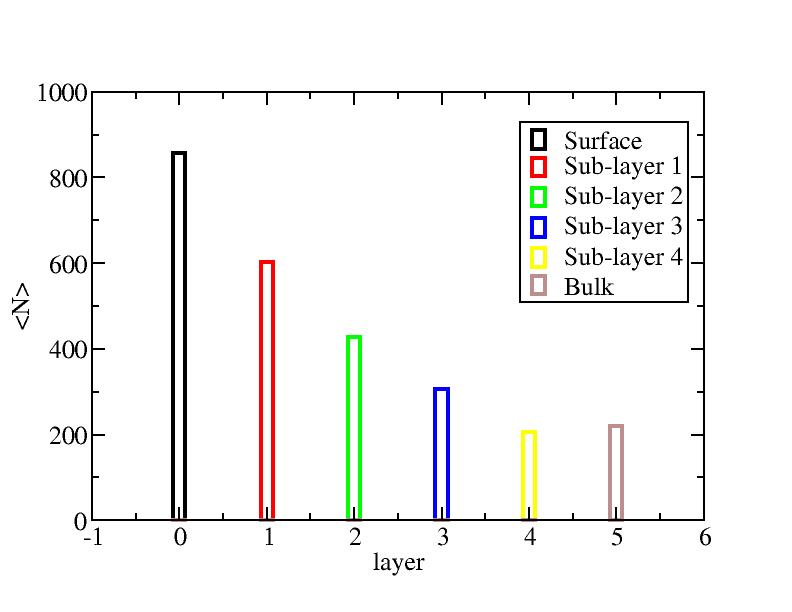
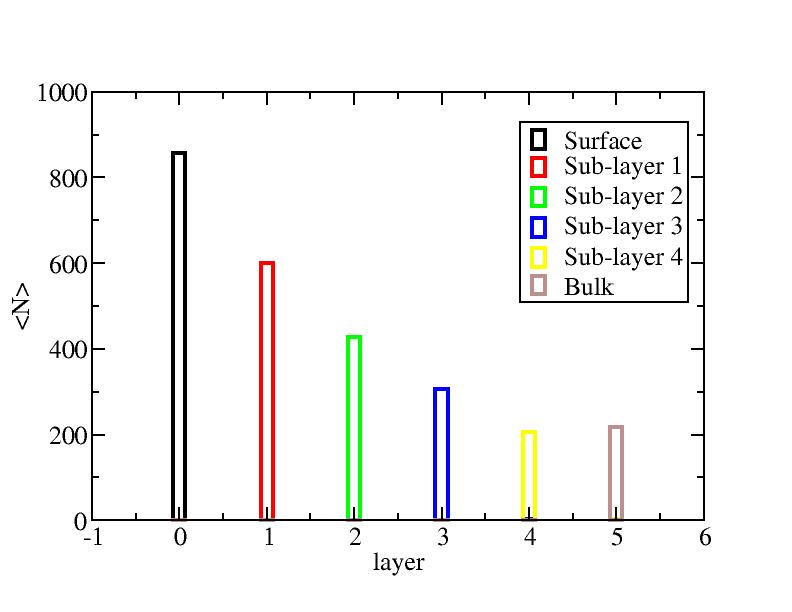
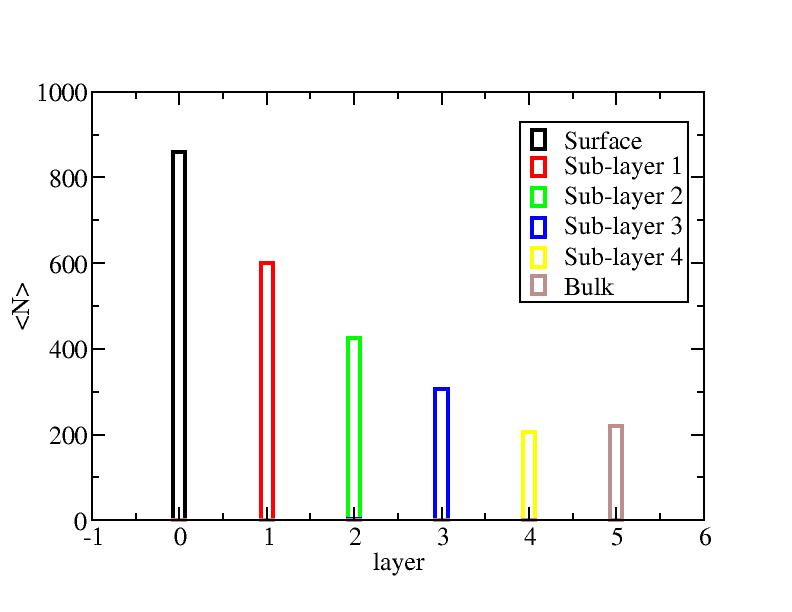
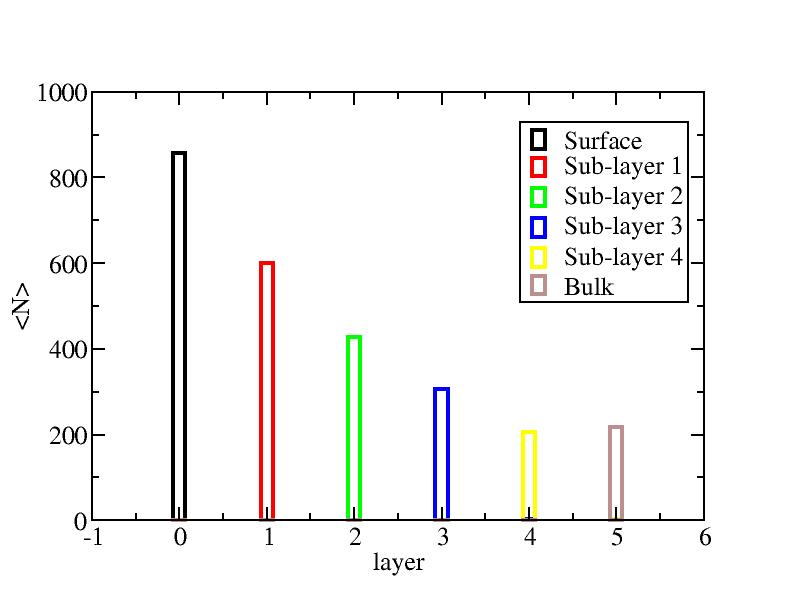
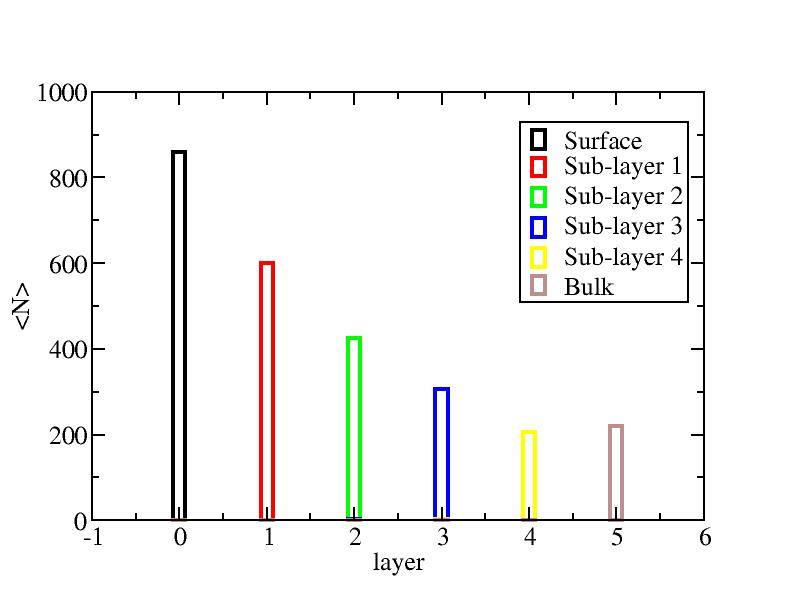
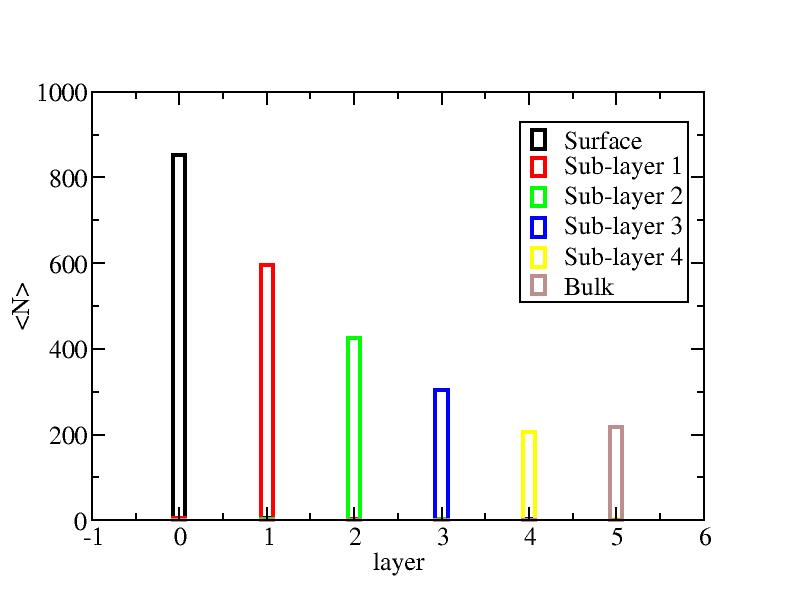
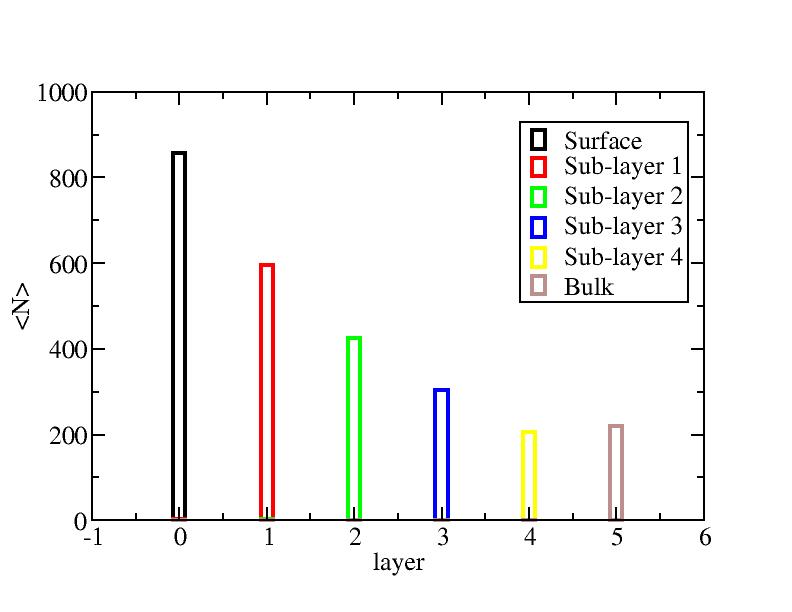
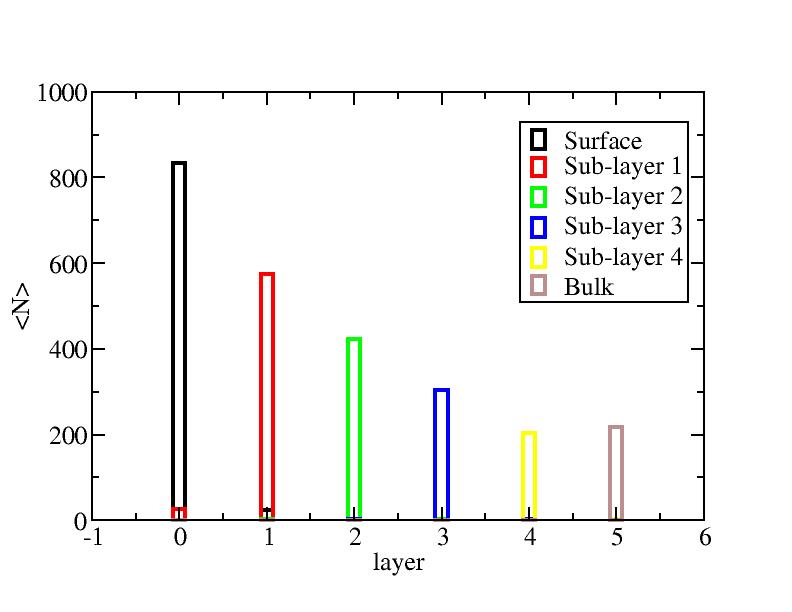
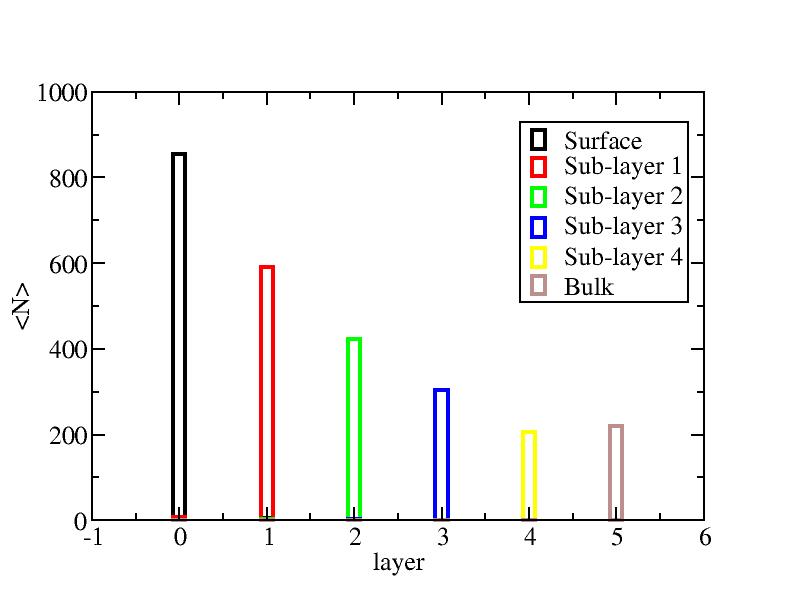
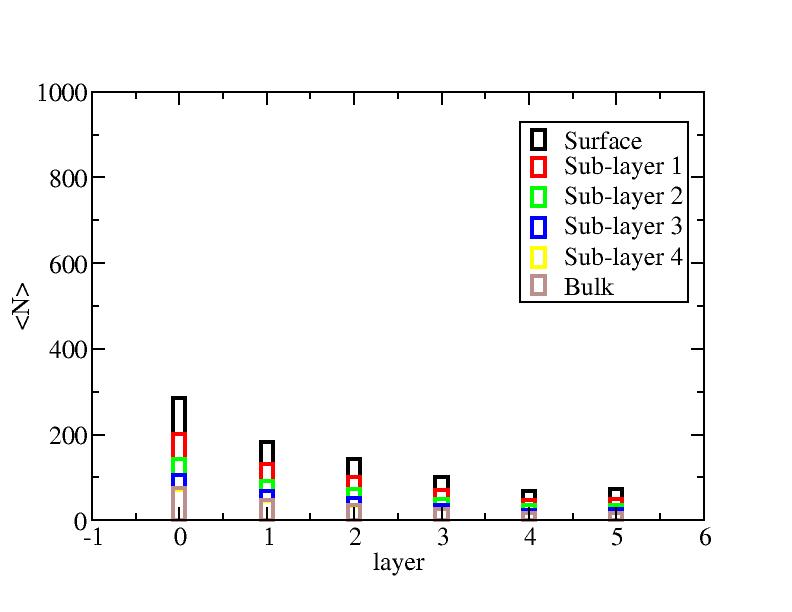
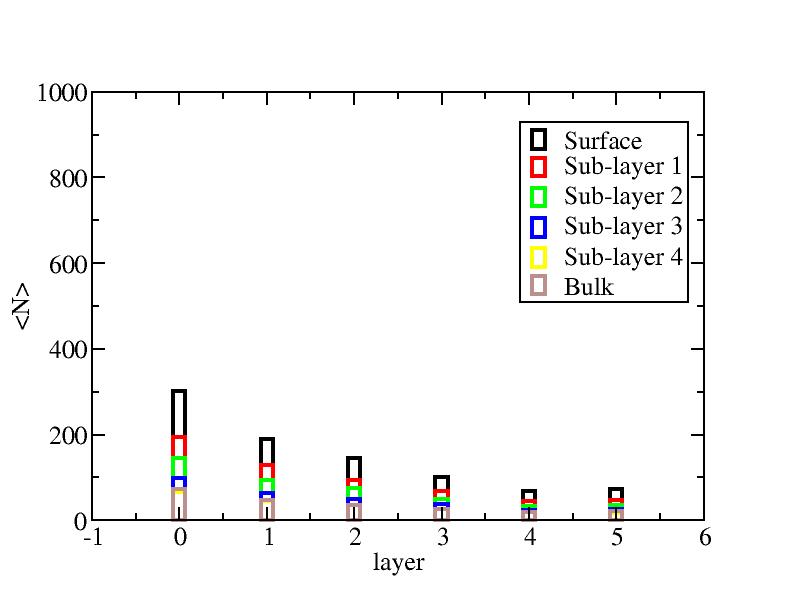
Surface layer, four sub-surface layers and bulk were distinguished for
the system at 5K (quenched by conjugate gradient minimization) and higher
temperature configurations (100K-1400K, 100 configurations for each temperature
level). The initial layer-numbers of atoms are define as:
|
Layer-number
|
Layer
|
|
0
|
Surface
|
|
1
|
Sub-layer 1
|
|
2
|
Sub-layer 2
|
|
3
|
Sub-layer 3
|
|
4
|
Sub-layer 4
|
|
5
|
Bulk
|
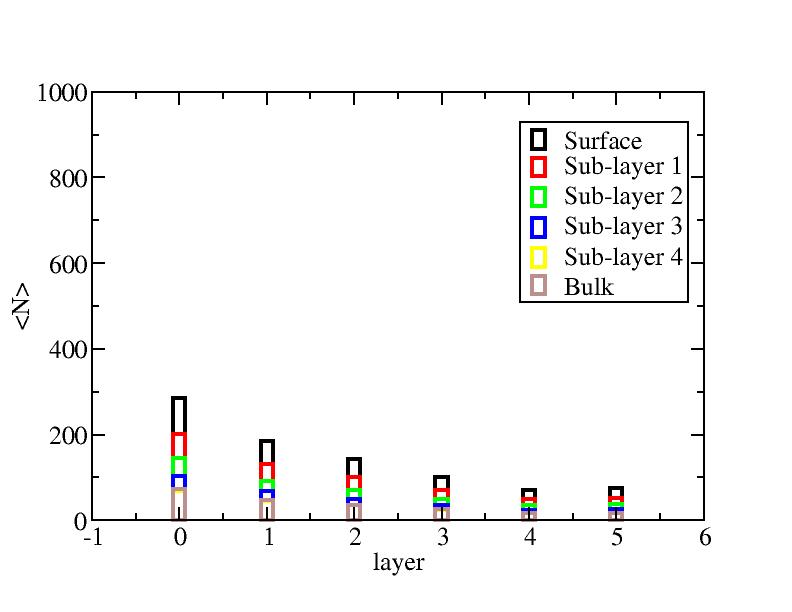
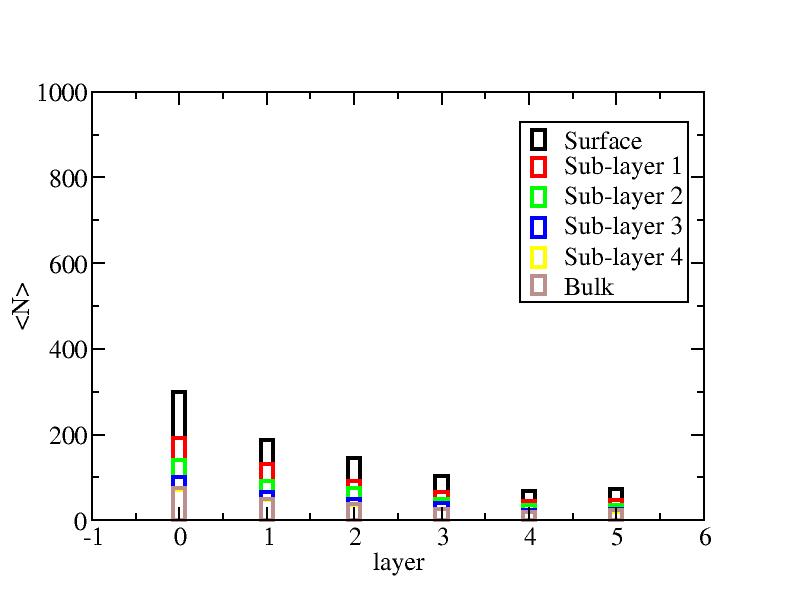
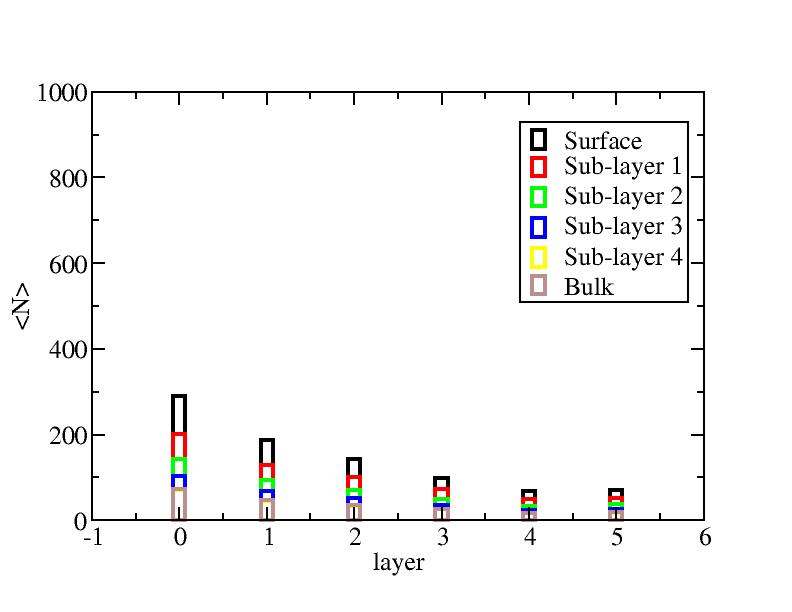
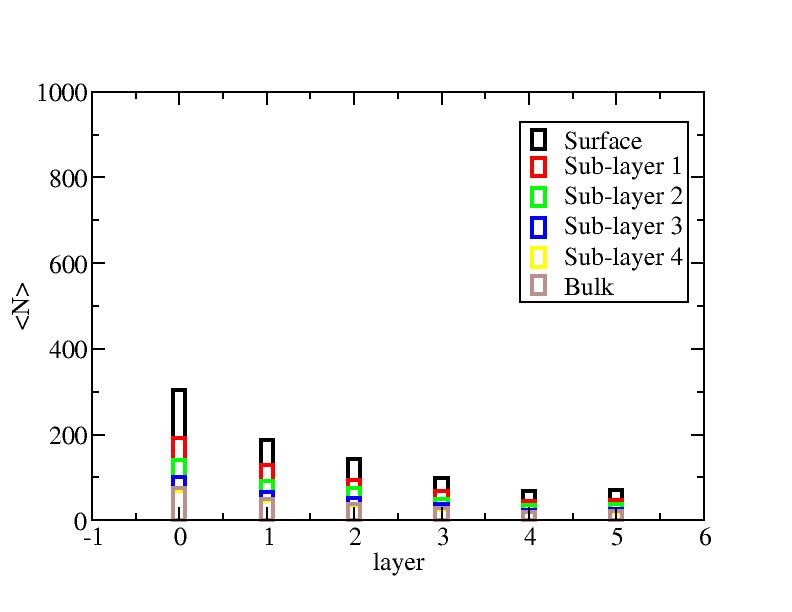
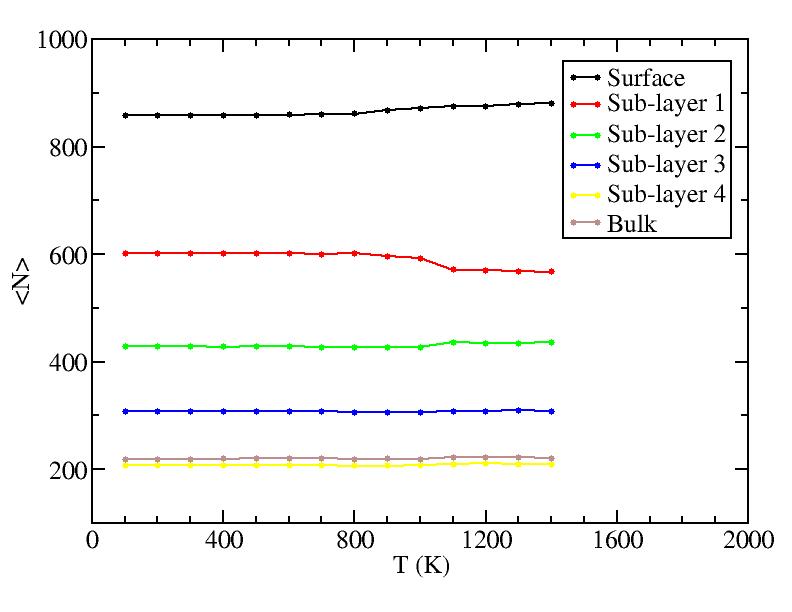
At each temperature, the layer-numbers of atoms were traced and averaged
over 100 configurations.
The right column is the results by defining the initial reference labels at
the start point of each temperature level individually.
Number of atoms on each layer
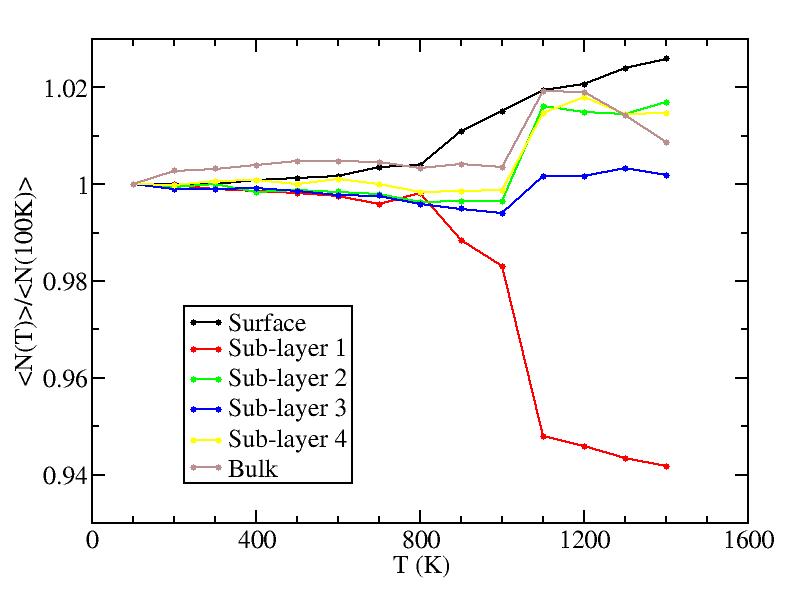
Change of number on each layer: N(T)/N(100K)
It may not mean anything because the difference of numbers are less than 30.
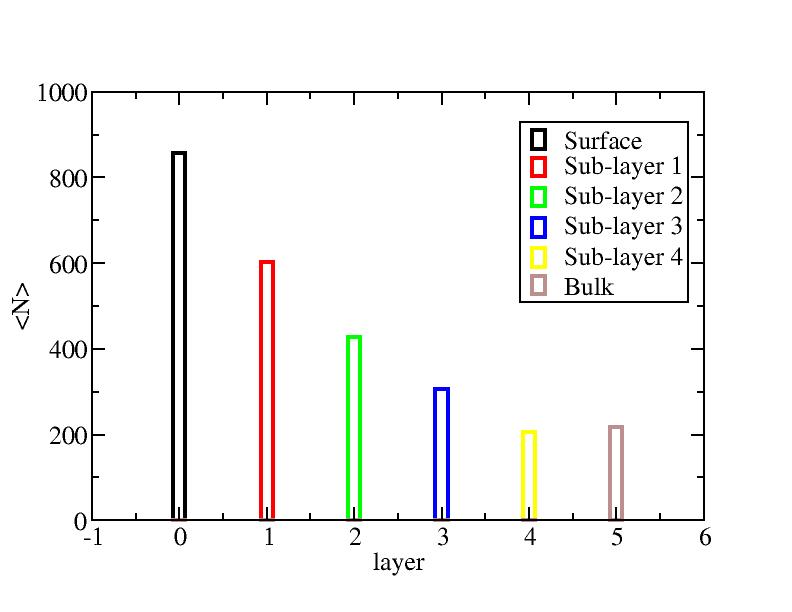
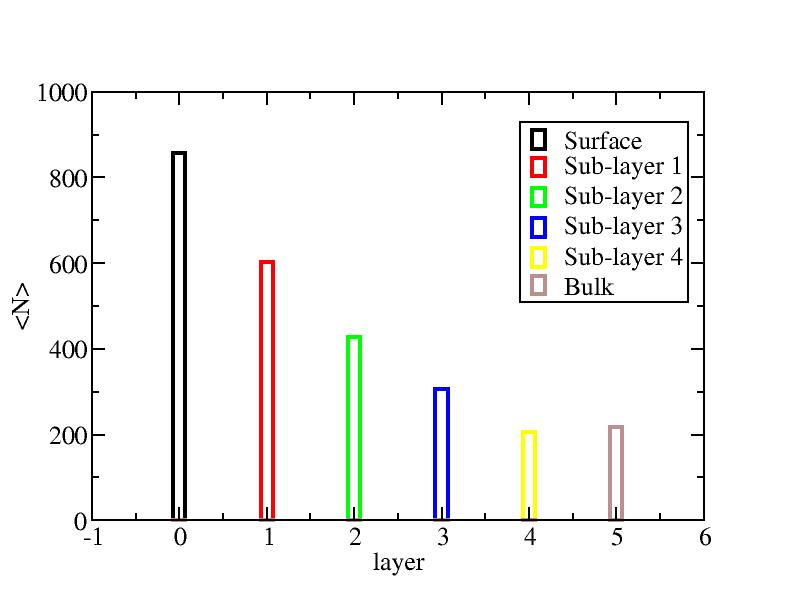
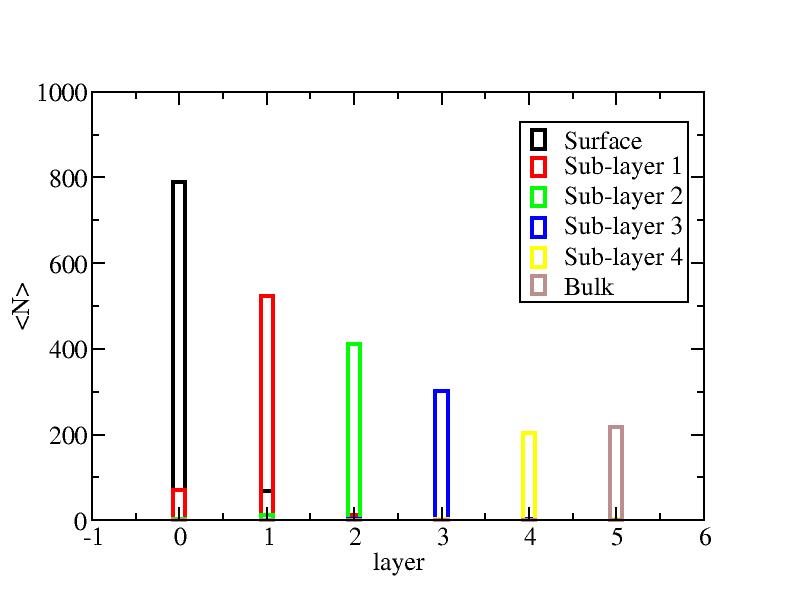
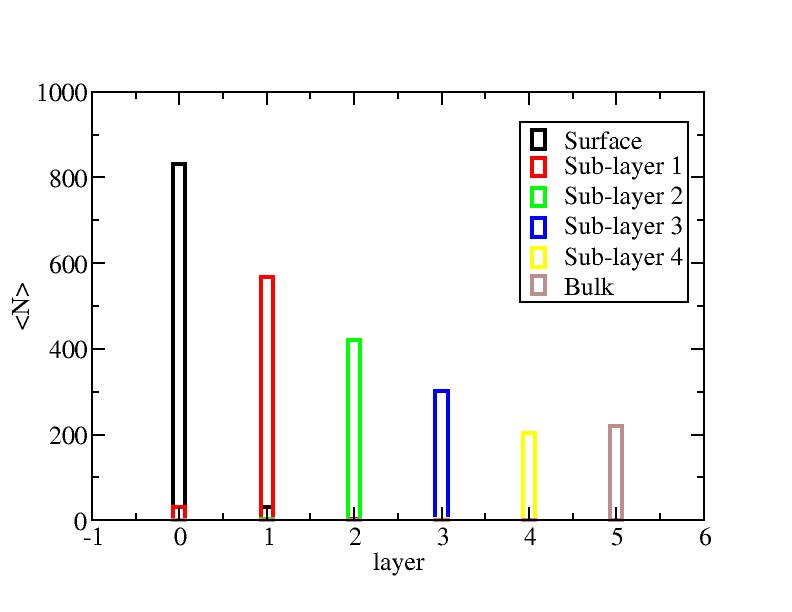
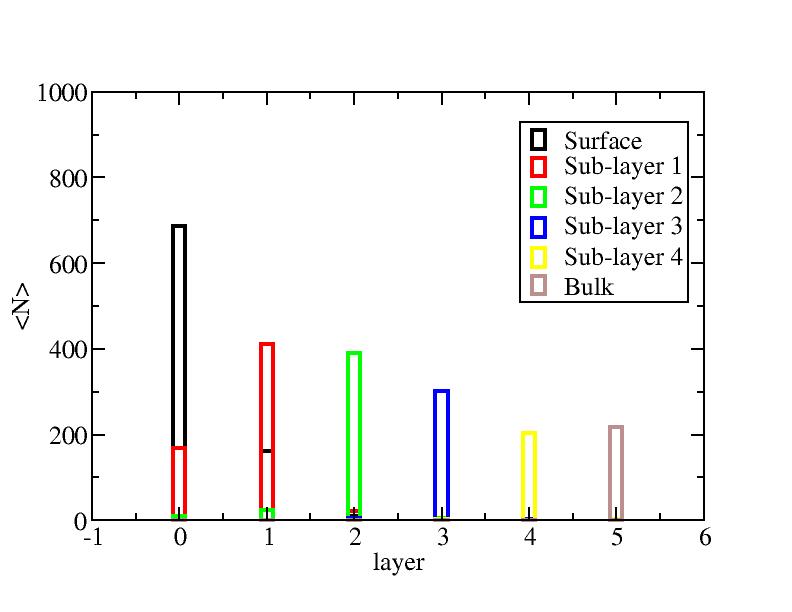
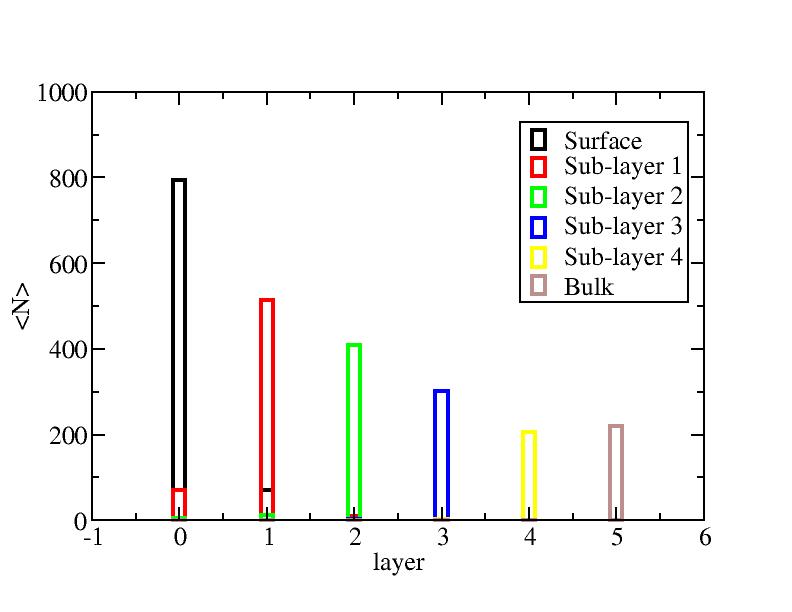
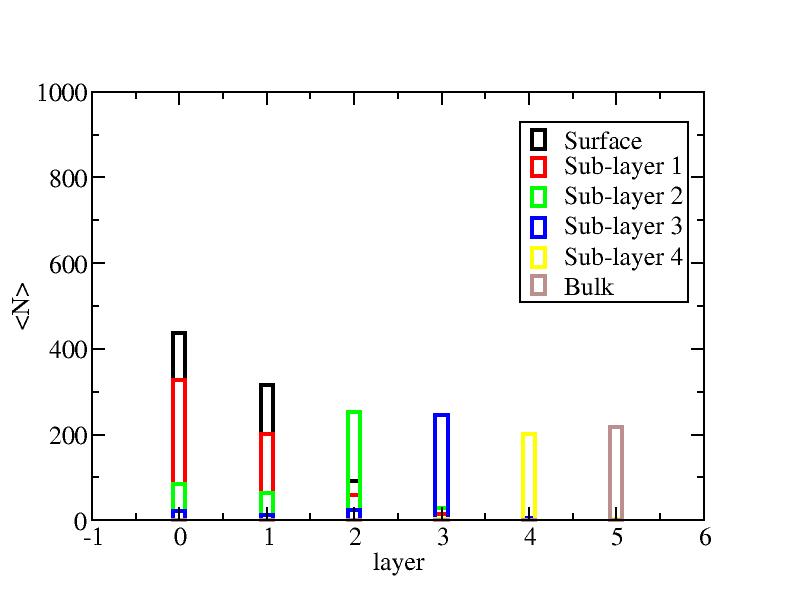
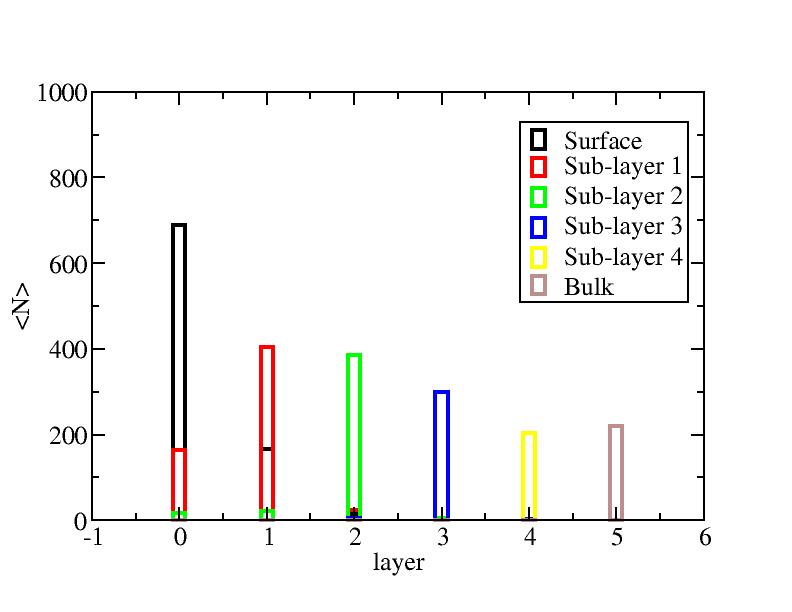
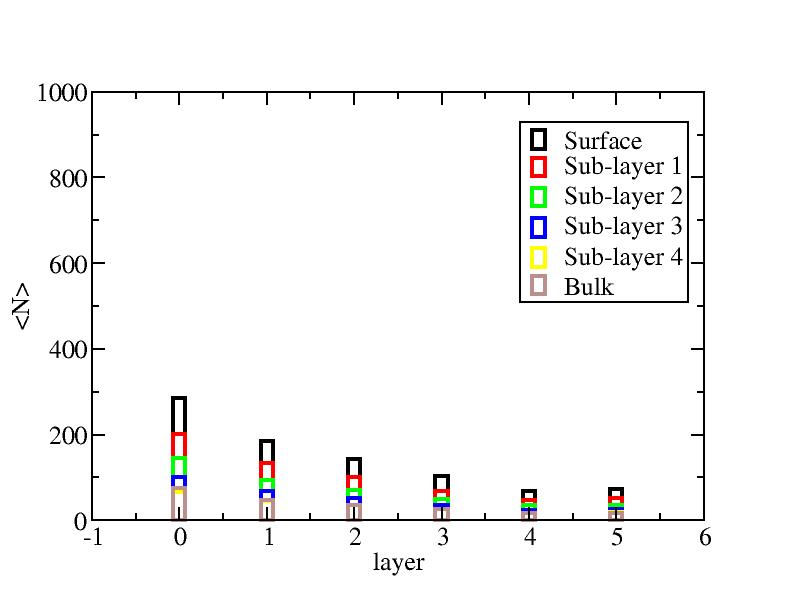
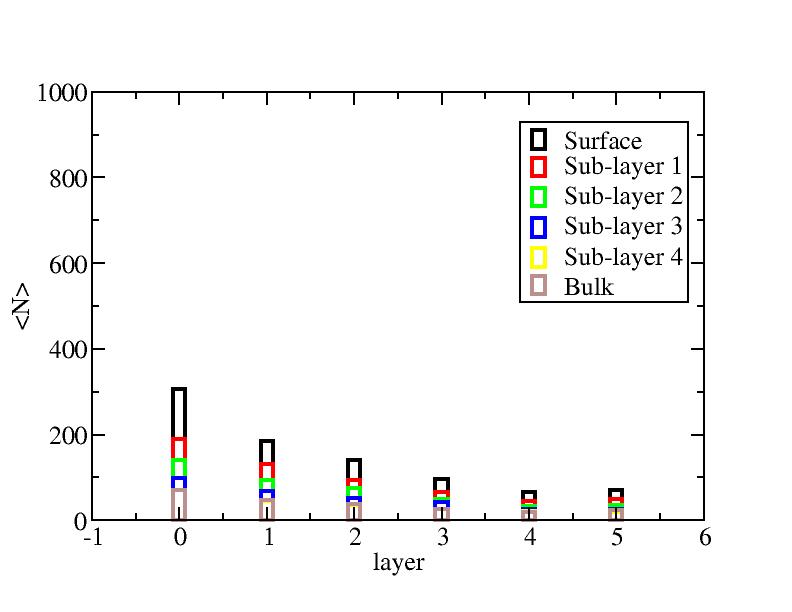
Distribution of atoms
The distribution of initial-layer-number-labeled atoms on different layers
at each temperature.
100K
200K
300K
400K
500K
600K
700K
800K
900K
1000K
1100K
1200K
1300K
1400K