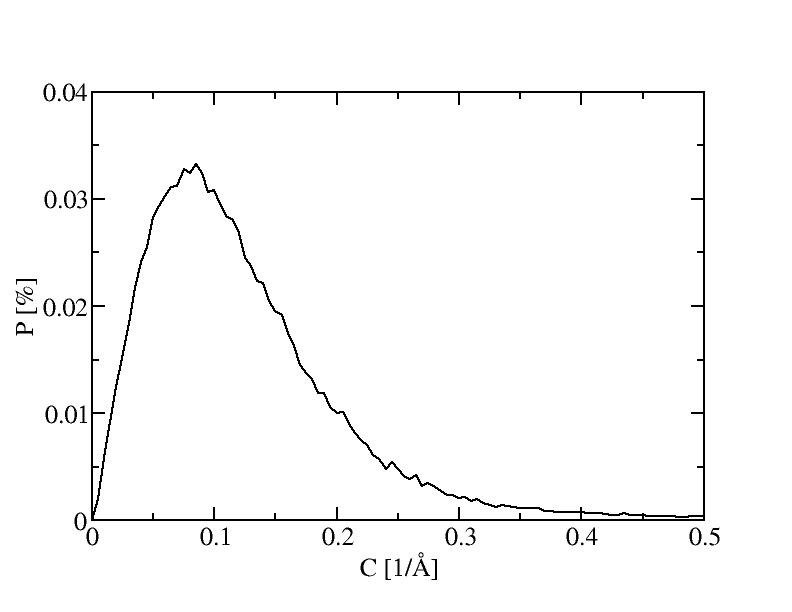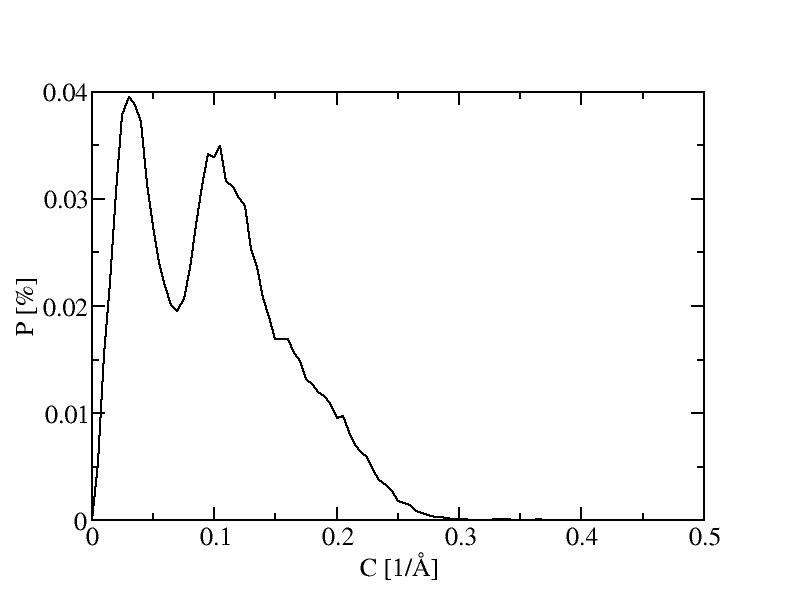
Let the positions of the two atoms be (x1, y1, z1) and (x2,y2,z2), the distance between them is:
L = sqrt( (x2-x1)2 + (y2-y1)2 + (z2-z1)2 )
Let the normal vectors be (nx1, ny1, nz1) and (nx2, ny2, nz2), theta be the angle between them,
cos(theta) = nx1*nx2 + ny1*ny2 + nz1*nz2
The radius of curvature can be calculated as:
r = L / ( 2*sin(theta/2) )
Because
cos(theta) = 1 - 2*sin2(theta /2)
The curvature is then
c = 1/r = sqrt( 2 * ( 1 - nx1*nx2 - ny1*ny2 - nz1*nz2 ) / ( (x2-x1)2 + (y2-y1)2 + (z2-z1)2 ) )
The histograms include 100 bins.
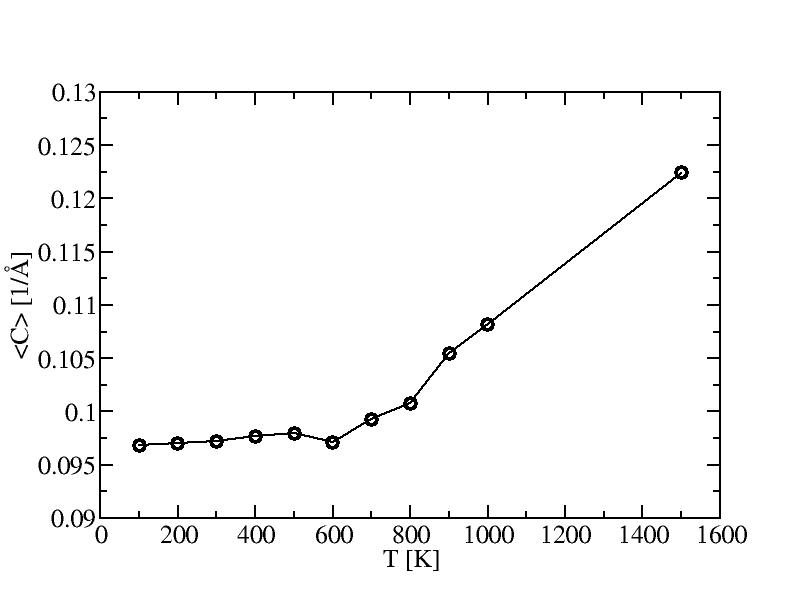
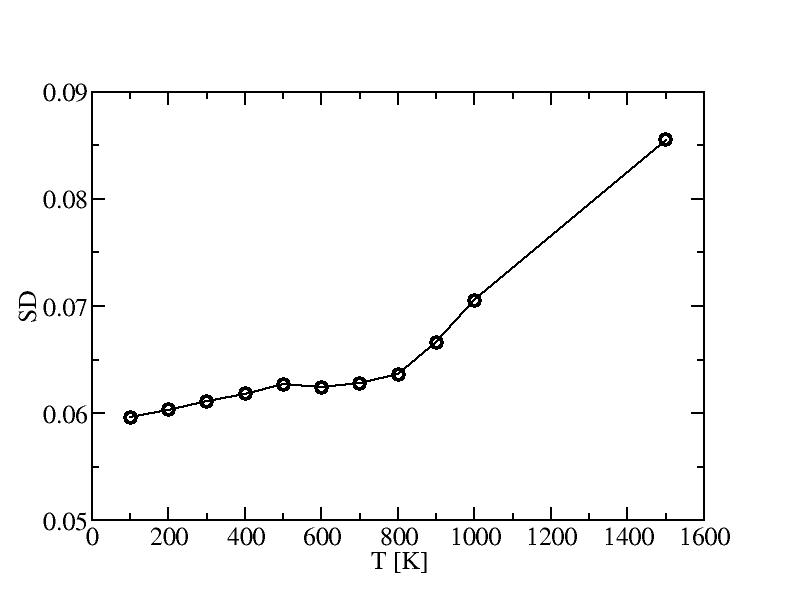
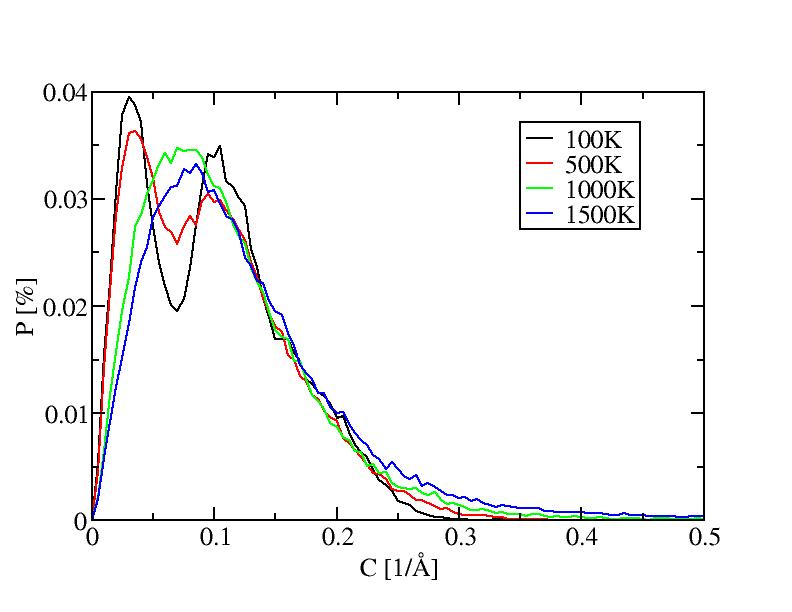
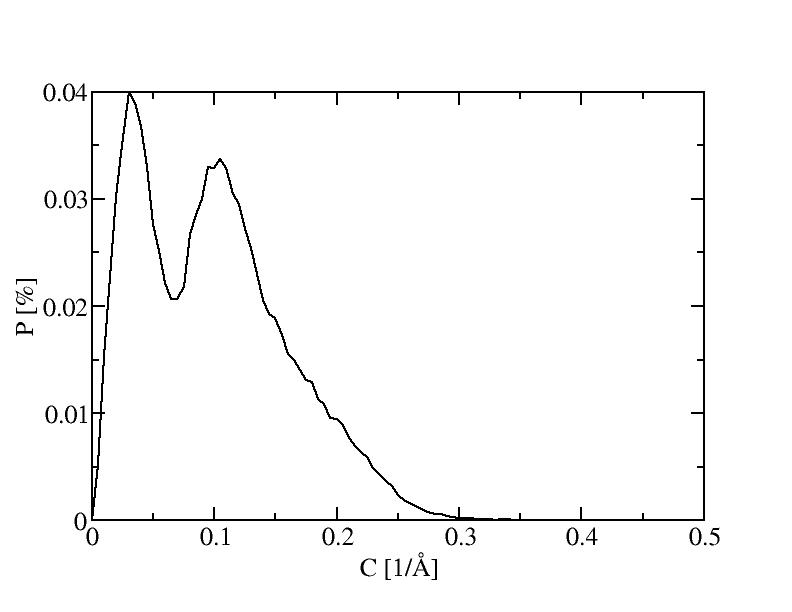
1) 100K

2) 200K
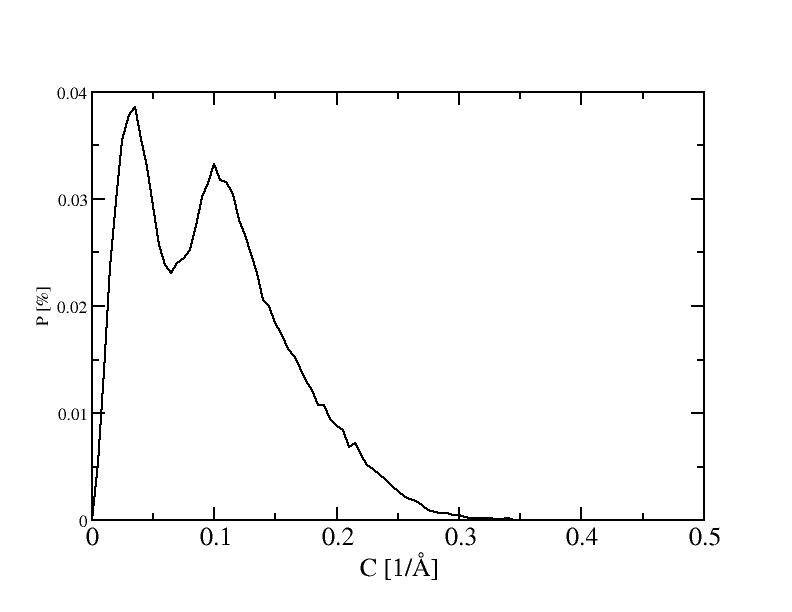
3) 300K
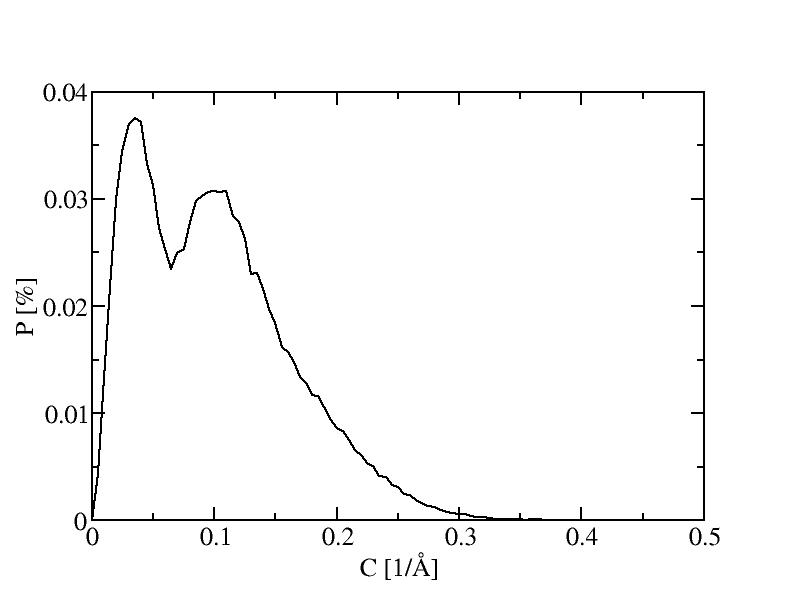
4) 400K
5) 500K
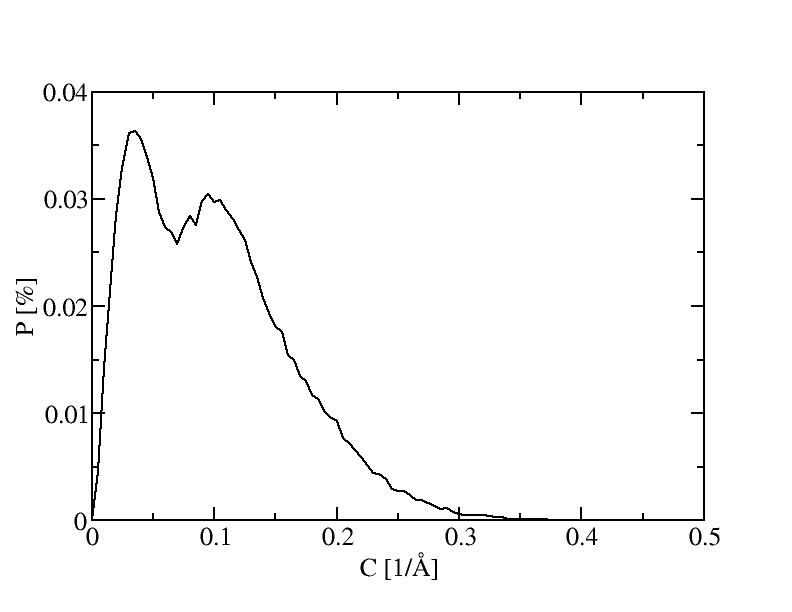
6) 600K
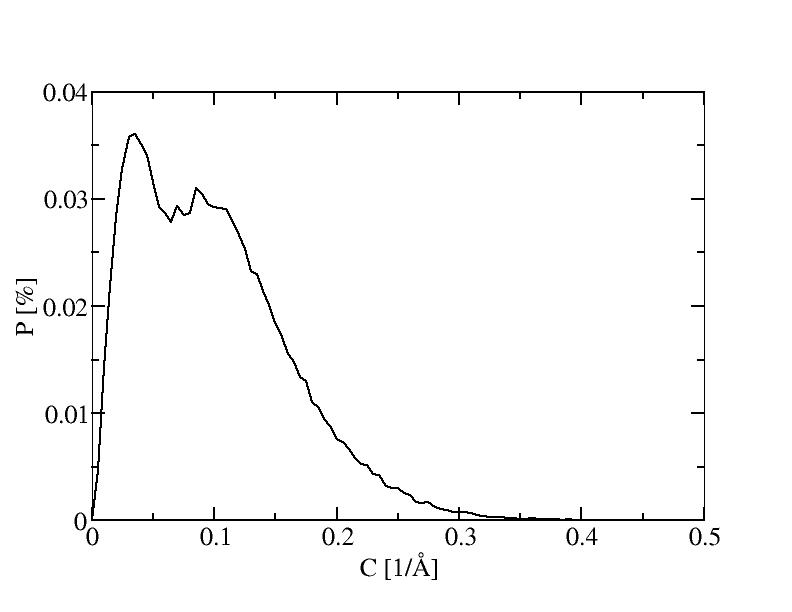
7) 700K
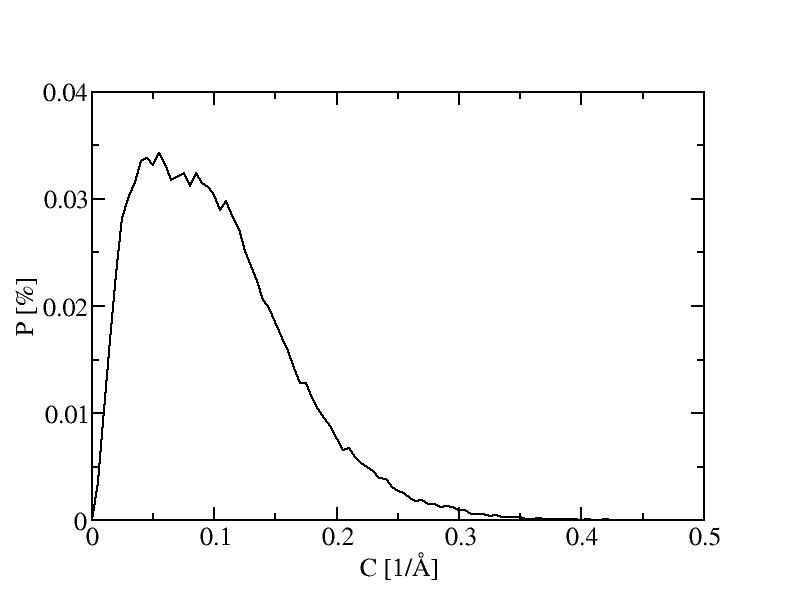
8) 800K
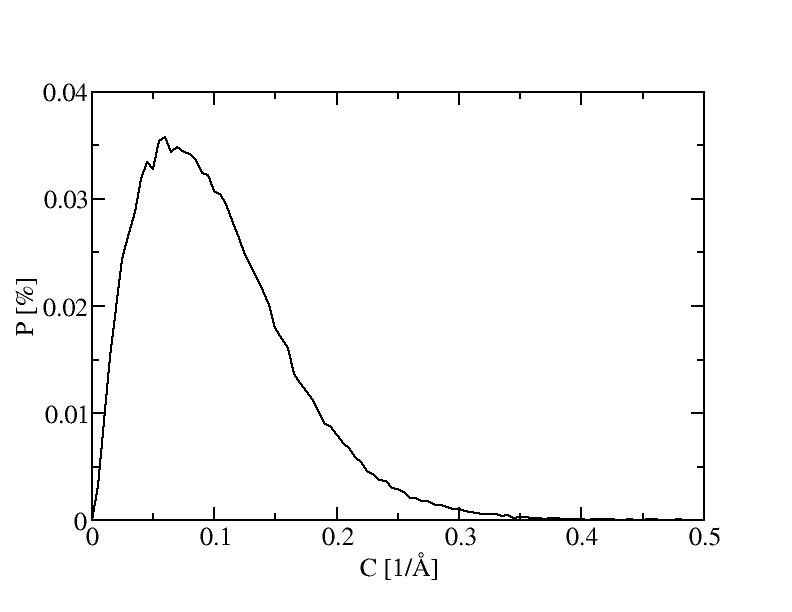
9) 900K
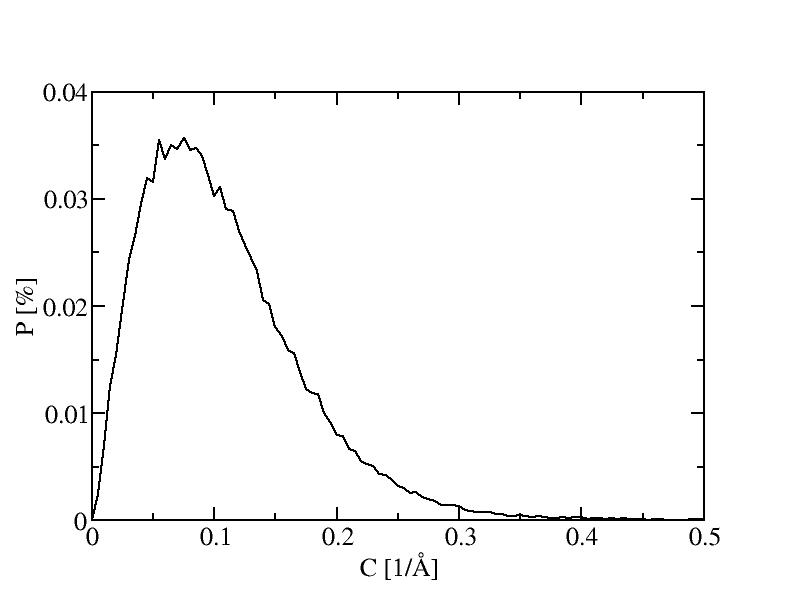
10) 1000K
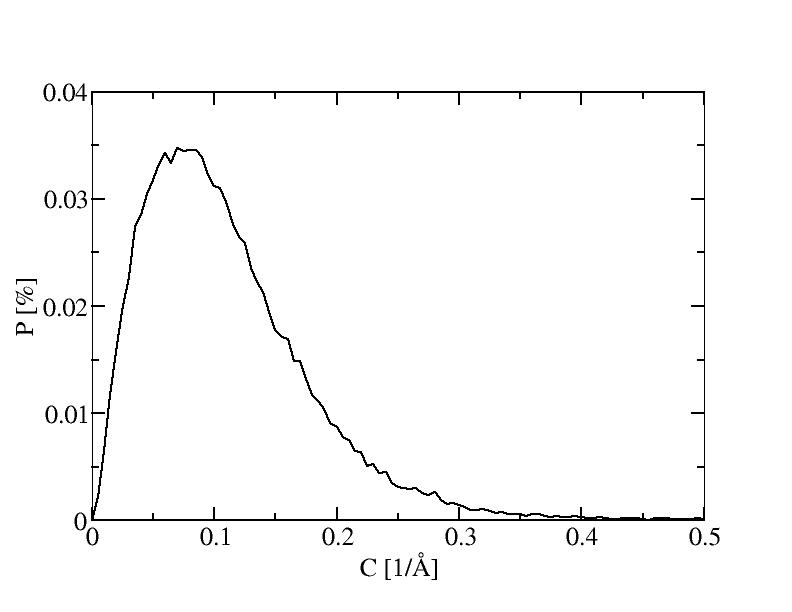
11) 1500K