November 14 2002
Physical properties of 603 atoms
Ansersen thermostat can generate the static properties very well but not
the dynamical properties due to its non-physical dynamics. In order to
calculate the diffusion coefficient and const-volume specific heat capacity,
the configurations cooled by Andersen thermostat have gone through constant-energy
MD to collect data. At each tempearture lever the equilibrium have run
for 10^6 steps (4.3ns). The average potential energy and bond order parameters
based on this simulation have also been calculated to compare with the
pictures on Octobor 24 2002.
According to the melting curve, the melting
temperature of 603 atoms should be around 870K.
Diffusion coefficient
The diffusion coefficient is given by
D = d< ( r(t1)-r(t0) )2 > / dt /
6
The diffusion coeffients for 879 atoms have been given in F. Ercolessi
et al. Phys. Rev. Lett. 66 911 (1991) as the following graph:
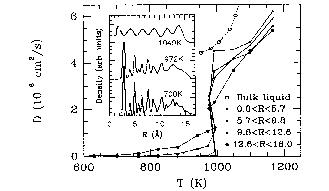
Our results are shown below:
Diffusion coefficient:
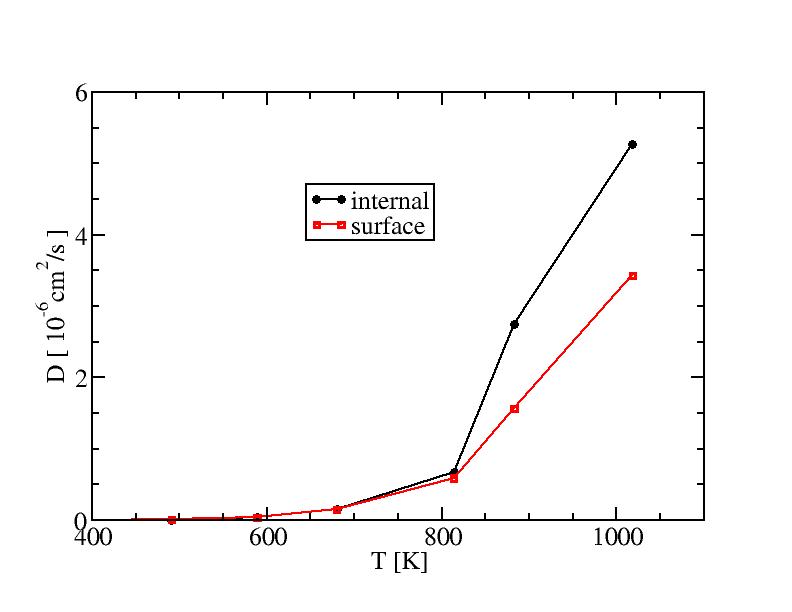
Mean square displacement of internal atoms:
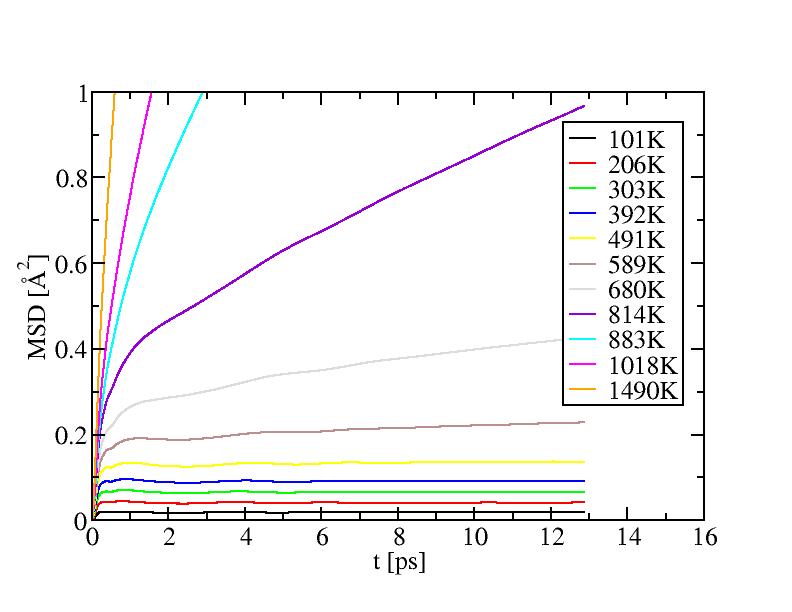
Mean square displacement of surface atoms:
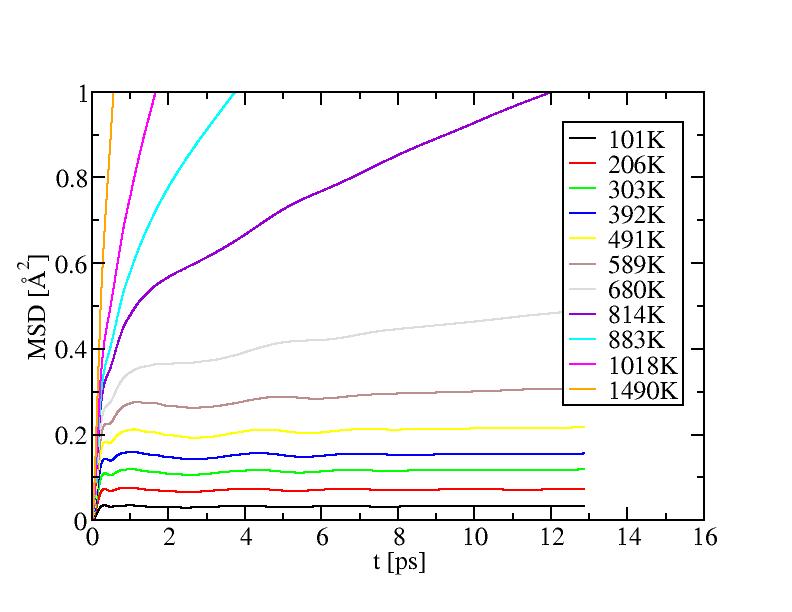
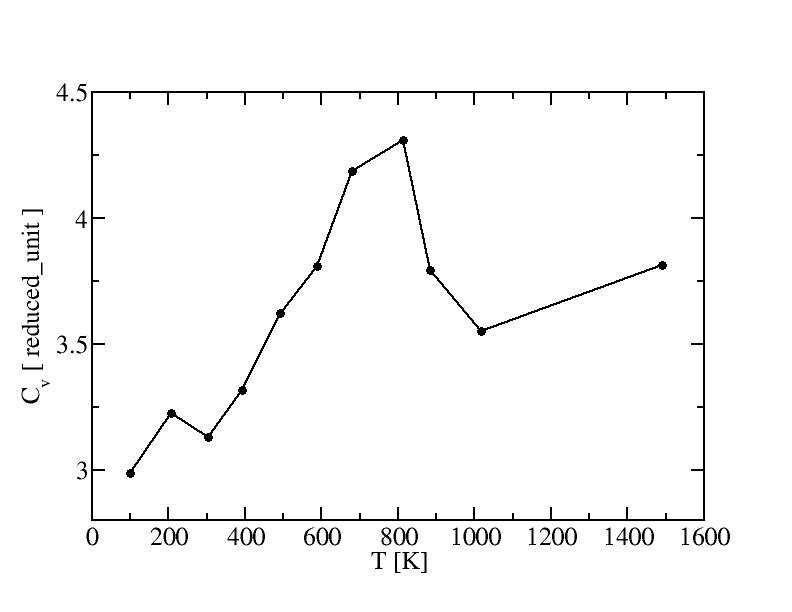
Constant-volume specific heat capacity
The constant-volume specific heat capacity can be calculated by the fluctuation
of kinetic energy K in microcanonical simulation:
<K2> - <K>2 = ( 1 - 3kB/(2Cv)
) * 3kB2T2 / (2N)
The experimental heat capacityof bulk gold at 300K is 129J/(Kg*K) =
4.2*10-23J/K per atom = 3.1 reduced unit.
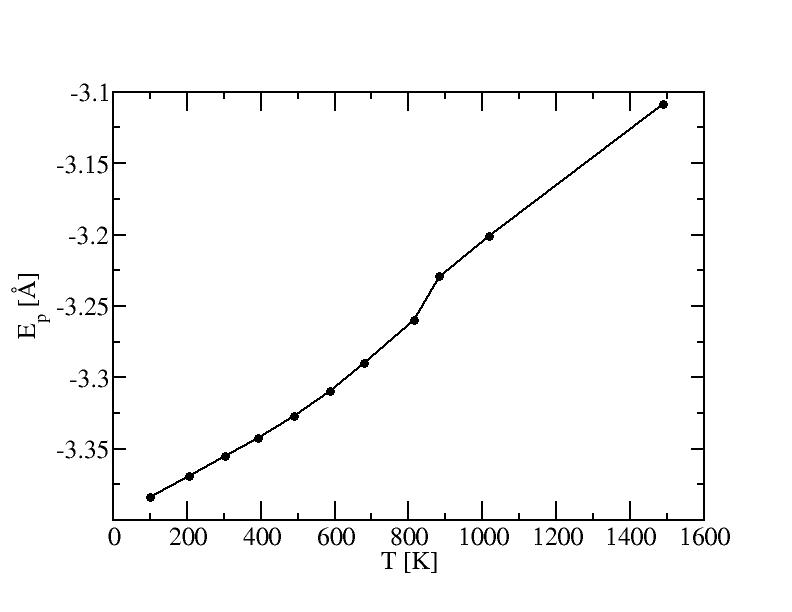
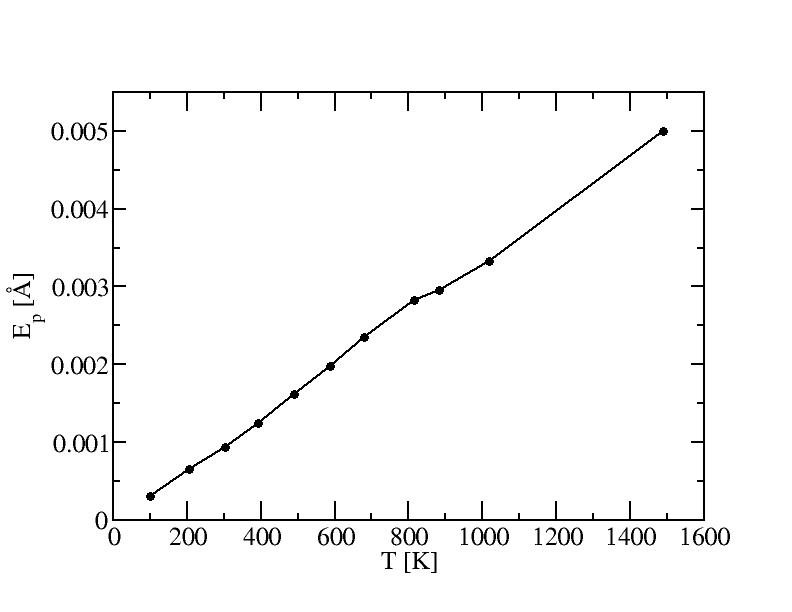
Average potential energies and their standard deviations
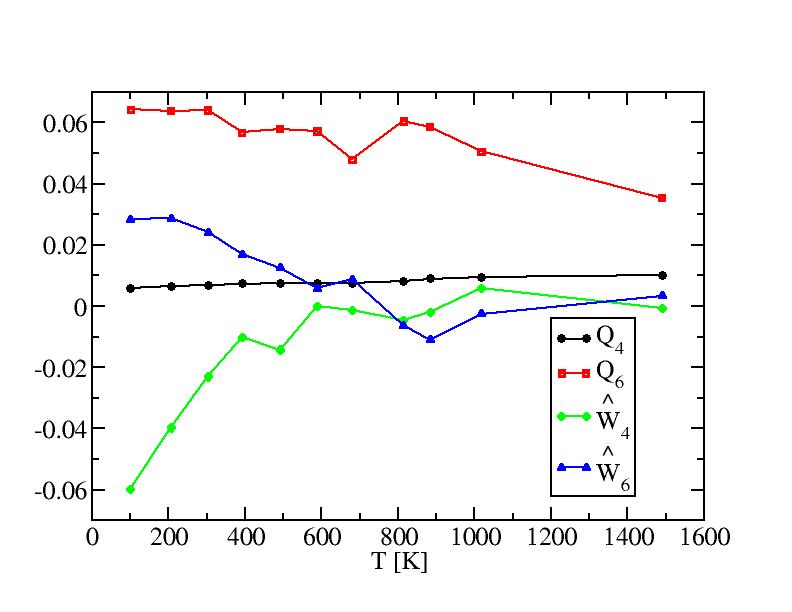
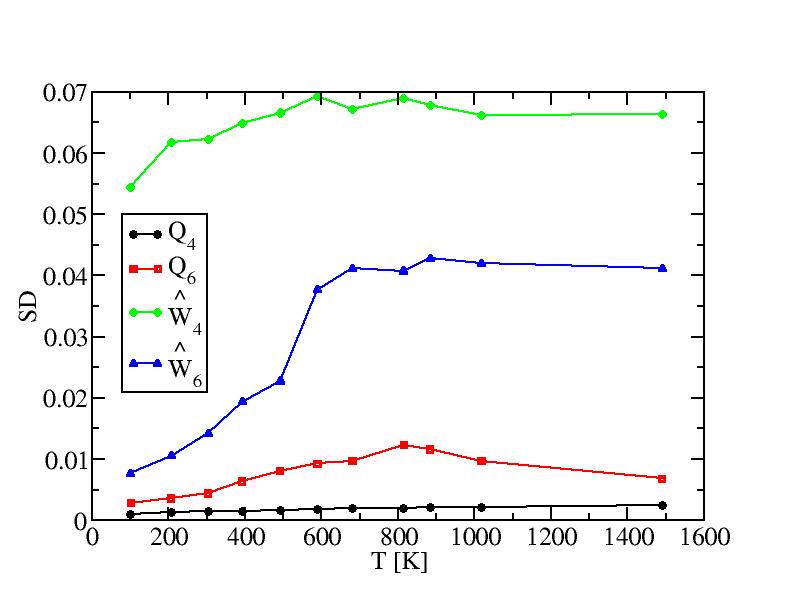
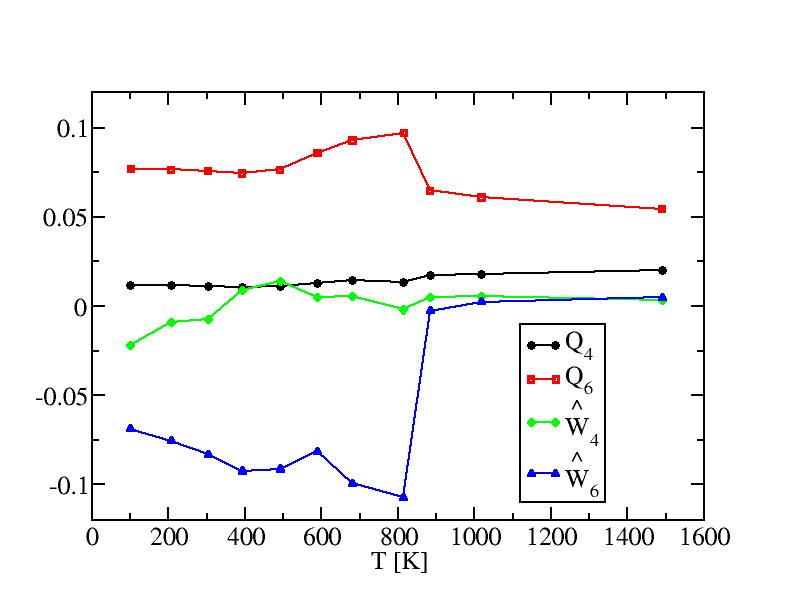
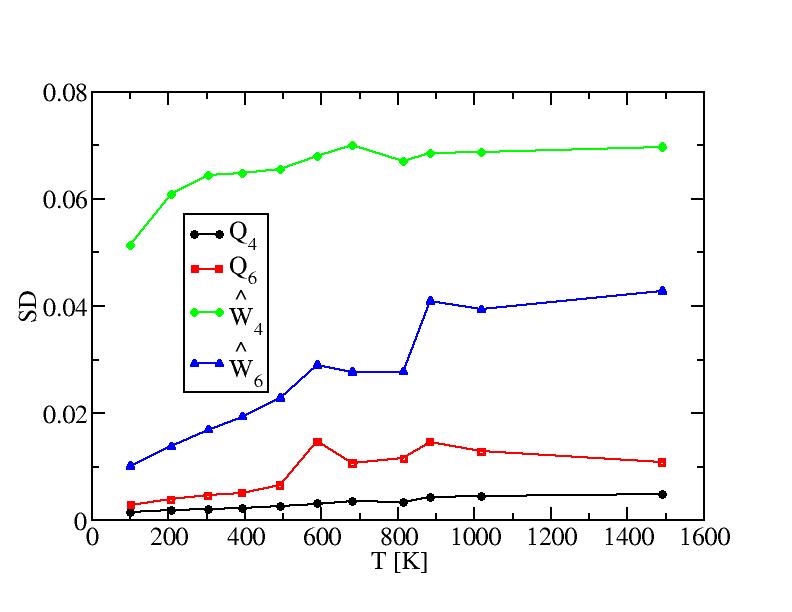
Bond order paramters and their standard deviations of internal atoms
Bond order paramters and their standard deviations of surface atoms