| |

Home
Contact Info
Course Info
Calendar
Homework
Lecture Notes
|
|
|
|
PHY 418: Statistical Mechanics I
Prof. S. Teitel stte@pas.rochester.edu ---- Spring 2007
Problem Set 2
Due Thursday, February 22, in the grader's mailbox by noon
- Problem 1 [10 points total]
A thermodynamic engine can be thought of as a cyclic process by which heat (TdS) pumped from a "hot" reservoir into a system (the engine) is converted into work (pdV) done by the engine. The most famous such example is the Carnot cycle. Another such cycle is the Otto cycle, schematically shown in the diagram below as a directed path in the volume-entropy plane. The Otto cycle is a rough approximation diagram below as a directed path in the volume-entropy plane. The Otto cycle is a rough approximation to the operation of a gasoline engine.
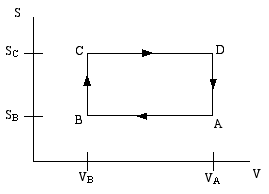
The gas is compessed adiabatically (constant entropy) in the step A to B. Then it is heated isochorically (constant volume) in the step B to C (this step corresponds to the combustion of the gas in the gas engine). The gas is then expanded adiabatically in step C to D (this is the power stroke).. Finally the gas is cooled isochorically to bring it back to its initial state; this is step D to A.
Step B to C extracts heat QBC from the "hot" reservoir to heat the gas, while step D to A returns heat QDA = - QAD to the "cold" reservoir (usually the ambient atmosphere). The difference is the work done by the engine.
If the engine efficiency is defined as ratio of the net heat converted to work, divided by the heat withdran from the hot reservoir
ε = (QBC - QAD)/QBC
then assuming the gas is an ideal gas, show that the efficiency of the Otto cycle is given by,
ε = 1 - (VB/VA)(Cp - CV)/CV
- Problem 2 [15 points total]
Consider a box of volume V. The box is split exactly in half by a thermally conducting imovable wall. Equal quantities of the same type of ideal gas fill each half of the box, and the system is in equilibrium. The total energy of the gas is fixed at ET.
a) Show that the pressure of the gas on each side is the same.
b) Using the formular derived in lecture for the number of states Ω(E) of an ideal gas at total energy E, find the most likely value for the energy of the gas on one side of the box.
c) Using Ω(E), if P(E) is the probability distribution for the gas on one side of the box to have energy E, show that the relative width (i.e. the width divided by the average) of P(E) is proportional to 1/N1/2, for sufficiently large N, where N is the number of particles in the gas. This shows that the fluctuation of E away from its average becomes negligibly small in the thermodynamic limit of N going to infinity.
- Problem 3 [25 points total]
Consider a system of N distinguishable non-interacting objects, each of which can be in one of two possible states, "up" and "down", with energies +ε and -&epsilon. Assume that N is large.
(a) Working in the microcanonical ensemble, find the entropy of the system S(E, N) as a function of fixed total energy E and number N (Hint: it is useful to consider the numbers N+ and N- of up and down objects).
(b) Using your result from (a) find the temperature T as a function of energy E and number N. Show that T will be negative if E>0.
(c) What happens if such a system (1) with T1<0 comes into thermal contact with another such system (2) with T2>0? Does T1 increase or decrease? Does T2 increase or decrease? In which direction does the heat flow?
(d) Working in the canonical ensemble, find the Helmholtz free energy A(T, N) as a function of temperature T and number N.
(e) Starting from A(T, N) of part (d), find the canonical entropy and express it as a function of the average energy E and number N. Show that your result agrees with your answer for part (a), in the large N limit.
- Problem 4 [10 points total]
In lecture we derived the canonical partition function QN(V,N) for the idea gas using a factorization method. One can also directly compute it by taking the Laplace transform of the microcanonical partition function Ω(E,V,N). Using our result from lecture,
| Ω(E,V,N) = [ |
V
h3 |
(2πmE)3/2 |
]N |
1
[(3N/2) - 1]! N! |
(Δ/E) |
compute directly the Laplace transform,
| QN(T,V) = |
∞
∫
0 |
dE
&Delta |
Ω(E,V,N)e-βE |
and show that you get the same result as found in lecture.
|
|