 |
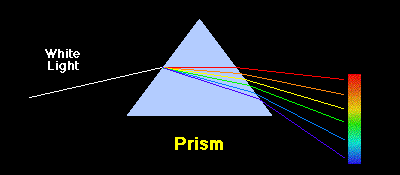
| Separation of light by a prism according to wavelength |
| Atomic Absorption and Emission Spectra |
As we have noted in the section on the Bohr atom, isolated atoms can absorb and emit packets of electromagnetic radiation having discrete energies dictated by the detailed atomic structure of the atoms. When the corresponding light is passed through a prism or spectrograph it is separated spatially according to wavelength, as illustrated in the following image.
 |
| Separation of light by a prism according to wavelength |
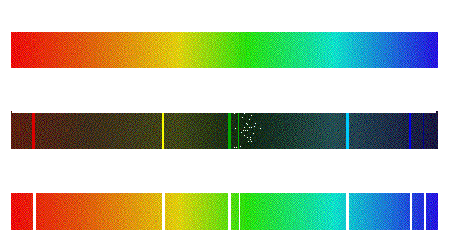 |
| Continuous, emission, and absorption spectra |
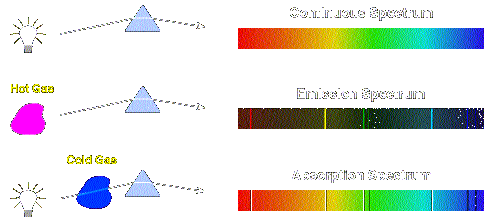 |
| Sources of continuous, emission, and absorption spectra |
A continuum spectrum results when the gas pressures are higher, so that lines are broadened by collisions between the atoms until they are smeared into a continuum. We may view a continuum spectrum as an emission spectrum in which the lines overlap with each other and can no longer be distinguished as individual emission lines. BLACKBODY IS AN EXAMPLE OF CONTINUUM EMISSION (energy at all frequencies).
An absorption spectrum occurs when light passes through a cold, dilute gas and atoms in the gas absorb at characteristic frequencies; since the re-emitted light is unlikely to be emitted in the same direction as the absorbed photon, this gives rise to dark lines (absence of light) in the spectrum.
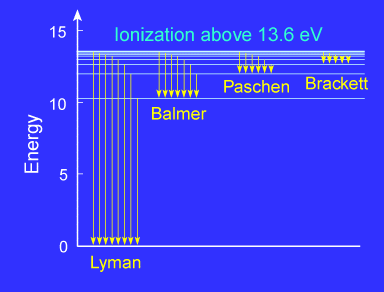 |
| Hydrogen emission series |
Because of the details of hydrogen's atomic structure, the Balmer Series
is in the visible spectrum and the Lyman Series is in the the UV.
The following image illustrates some of the transitions in the Balmer series.
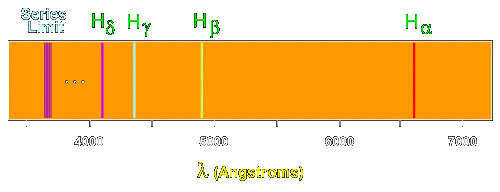 |
| The Balmer spectrum of hydrogen |
The Balmer lines are designated by H with a greek subscript in order of decreasing wavelength. Thus the longest wavelength Balmer transition is designated H with a subscript alpha, the second longest H with a subscript beta, and so on.
In addition to spectra associated with atoms and ions, molecules can interact with electromagnetic radiation and give rise to characteristic spectra. Because of basic atomic and molecular structure, the spectra associated with molecules typically involve infrared wavelengths. In addition, because molecules are usually fragile, molecular spectra are important mostly in objects that are relatively cool such as planetary atmospheres, the surfaces of very cool stars, and various interstellar regions.