|
|

Home
Contact
Info
Course
Info
Calendar
Homework
Lecture
Notes
|
|
|
|
Physics 418: Statistical Mechanics I
Prof. S. Teitel stte@pas.rochester.edu ----- Spring 2005
Problem Set 4
Due Tuesday, March 15, in lecture
- Problem 1 [20 points total]
Consider a classical gas of N indistinguishable non-interacting
particles with ultrarelativistic energies, i.e. their kinetic energy -
momentum relation is given by  = pc, with c the speed of light and p the
magnitude of the particle's momentum. = pc, with c the speed of light and p the
magnitude of the particle's momentum.
(a) Compute the canonical partition function for this system. [5 points]
(b) Show that this system obeys the usual ideal gas law, pV =
NkBT. [5 points]
(c) Show that the total average energy is, E = 3NkBT
(and hence with (a), E/V = 3P). [5 points]
(d) Show that the ratio of specific heats is, Cp/CV =
4/3. [5 points]
- Problem 2 [20 points total]
Consider a three dimensional classical ideal gas of atoms of mass m, moving in a potential energy
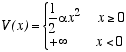
The infinite potential for x < 0 may be viewed as a rigid wall filling the y-z plane at x=0. The atoms, therefore, are attracted to this wall, but they move freely in the y and z directions. Let T be the temperature and n = N/A be the total number of atoms per unit area of the wall.
a) calculate the local atomic density n(x) (number of atoms per unit volume) as a function of the distance x from the wall. Note that
| n= | 
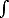
0 | n(x) dx |
[4 pts]
b) Show that the ideal gas law (relating pressure, temperature, and density) continues to hold locally everywhere. [4 pts]
c) Find the pressure that the atoms exert on the wall. How does this pressure vary with temperature? [4 pts]
d) Calculate the energy and specific heat per unit area of the wall. [4 pts]
e) Find the chemical potential of the gas. [4 pts]
- Problem 3 [25 points total]
Consider a classical gas of very weakly interacting molecules of different species.
There are Ni molecules of species number i, i = 1, 2, ..., m.
The molecules of
different species may have different masses and different internal degrees of
freedom (such as vibrational or rotational modes), however you may assume
that the molecules do not interact with each other. The molecules of a given species are indistiguishable from each other.
(a) Show that the canonical partition function for the gas has the form
Q = Q(1)Q(2)....Q(m)
where
Q(i) =
[Q1(i)]Ni/Ni!
and Q1(i) is the single particle partition function
for molecules of species i. [5 points]
(b) Using the result in (a), show that the total pressure of
the gas is the sum of the pressures that each of the species of
molecule would have on its own (total pressure is the sum of
the "partial pressures"). Similarly show that the total entropy
of the gas is the sum of the entropies that each species would have
on its own. [3 points]
(c) By taking the appropriate derivative of the total Helmholtz
free energy of the gas, compute the chemical potential µi
of species i. Express your answer in terms of Q1(i).
[3 points]
(d) Assume that the molecules are free (i.e. not in any external potential,
except for their confinement to a box of volume V). Show that
Q1(i) can be written as
Q1(i) = Vq1(i)(T) where
q1(i) depends only on temperature T. [3 points]
(e) Suppose that the species of molecules undergo the chemical reaction
a1A1 + ... +
ajAj <--> aj+1Aj+1 + ... +
amAm
where ai is the number of molecules of species Ai
(i = 1,...,j) that combine to create ak molecules of species
Ak (k = j+1,...,m). As discussed in lecture, the equilibrium
number of molecules of each species will be determined by the condition
a1µ1 + ... + ajµj
= aj+1µj+1 + ... + amµm
(make sure you understand where this result comes from!).
Use the above, and the results of the previous parts,
to show that the equilibrium concentrations of the species
are given by
([n1]a1
[n2]a2...[nj]aj
)/([nj+1]aj+1...[nm]am
) = K(T)
where ni is the concentration of species i,
ni = Ni/V, and K(T) is a
function of temperature only. Derive an expression for K(T) in terms
of the q1(i)(T). The above result is known as the
"law of mass action". [4 points]
(f) Consider the reaction
A + B <--> C
Suppose that an energy Eo is released when A and B combine to
form C, i.e. Eo is the binding energy of the molecule C when
compared to its separated constituents A and B. Assume that A, B, and C
are free point particles (i.e. no internal degrees of freedom are excited).
By explicitly evaluating the single particle partition function of the three
species, determine the function K(T). Remember, you must properly include the
binding energy Eo of molecule C when you compute its partition
function. [4 points]
(g) Suppose that initially there are equal concentrations of A and B,
nA = nB = no, while the concentration of C
is initially nC = 0. Find the resulting equilibrium concentrations
of A, B and C, in terms of no and the function K(T). [3 points]
|

