Theoretical Statistical and Condensed Matter Physics
Structural Stability of Gold Nanoclusters
Gold clusters consisting of tens to thousands of atoms have unique optical and mechanical properties and hold great promise as building blocks for nanobio-electronic devices, catalysts, and sensors. One reason leading to different behavior in such nanometer sized clusters, as compared to bulk materials, is their large surface-to-volume ratio. Surface and bulk free energies become comparable, an effect that plays a crucial role in the determining the shape and structure of such nanoclusters, and their stability against thermal fluctuations. Using the phenomenological "glue" potential to model the many-body interactions between gold atoms, we have used molecular dynamic simulations to study the structure and stability of gold nanoclusters under a variety of conditions. This work was carried out in collaboration with former graduate student Yanting Wang and Prof. Christoph Dellago of the University of Vienna.
Equilibrium Shape
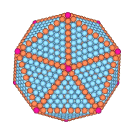 For macroscopically large samples, where surface effects are negligible, the equilibrium structure of simple materials is found to be a perfect periodic lattice. For gold, this periodic lattice is the face centered cubic crystal (fcc) structure. For gold nanoclusters of a few thousand atoms, however, we find that surface free energy is so important that the equilibrium structure that forms upon cooling a liquid droplet is no longer a pure periodic lattice, but rather is described as follows. Twenty tetrahedrally shaped pieces combine together to form a structure with icosahedral shape, as shown to the left. Each tetrahedron has an internal fcc structure with four ⟨111⟩ surface facets. When the tetrahedra combine to form the icosahedron, the fcc structure of each piece has a different global orientation, leading to strain-inducing twin planes where the surfaces of the tetrahedra, internal to the icosahedral structure, meet. However the cost in free energy due to these internal twin planes is more than compensated for by the reduction in surface free energy achieved by having the icosahedron covered entirely by ⟨111⟩ surface facets; the ⟨111⟩ surface facet of gold is known to be the most stable of all surface planes, remaining ordered up until the bulk melting temperature. As the icosahedral cluster is heated back towards melting, we find that the atoms along the edges of the cluster become mobile, leading to a rounding of the shape and a shrinking of the size of each facet. Just below the bulk melting temperature, the cluster shape becomes almost spherical, but the internal icosahedral structure remains stable. For further details, see Chem. Phys. Lett. 394, 257 (2004) and J. Chem. Phys. 122, 214722 (2005).
For macroscopically large samples, where surface effects are negligible, the equilibrium structure of simple materials is found to be a perfect periodic lattice. For gold, this periodic lattice is the face centered cubic crystal (fcc) structure. For gold nanoclusters of a few thousand atoms, however, we find that surface free energy is so important that the equilibrium structure that forms upon cooling a liquid droplet is no longer a pure periodic lattice, but rather is described as follows. Twenty tetrahedrally shaped pieces combine together to form a structure with icosahedral shape, as shown to the left. Each tetrahedron has an internal fcc structure with four ⟨111⟩ surface facets. When the tetrahedra combine to form the icosahedron, the fcc structure of each piece has a different global orientation, leading to strain-inducing twin planes where the surfaces of the tetrahedra, internal to the icosahedral structure, meet. However the cost in free energy due to these internal twin planes is more than compensated for by the reduction in surface free energy achieved by having the icosahedron covered entirely by ⟨111⟩ surface facets; the ⟨111⟩ surface facet of gold is known to be the most stable of all surface planes, remaining ordered up until the bulk melting temperature. As the icosahedral cluster is heated back towards melting, we find that the atoms along the edges of the cluster become mobile, leading to a rounding of the shape and a shrinking of the size of each facet. Just below the bulk melting temperature, the cluster shape becomes almost spherical, but the internal icosahedral structure remains stable. For further details, see Chem. Phys. Lett. 394, 257 (2004) and J. Chem. Phys. 122, 214722 (2005).
|
Nanorods
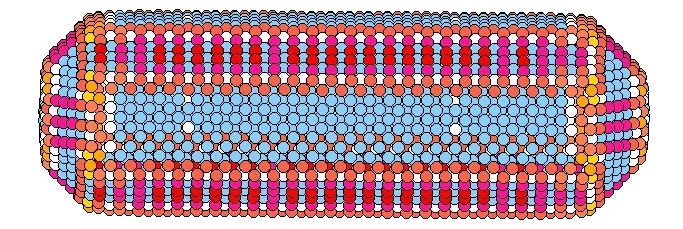 For macroscopically large samples, the crystal shape is generally determined by the growth process, rather than a true minimization of the free energy, due to the extremely long times needed for atoms to diffuse across the sample dimensions. For nanosized clusters, however, this is not necessarily so, and rod shaped gold nanoclusters have been observed to buckle, bulge, and transform to shapes with smaller aspect ratios, upon heating. The ability to form stable clusters of specified shape is crucial to the integration of such nanoclusters in device applications. We find that the stability of such gold nanorods of a few thousand atoms is crucially determined by the orientation of their surface facets. When the long axis of the rod has substantial surface area covered by either ⟨100⟩ or ⟨110⟩ oriented facets (as in the picture to the left), we find that the rod undergoes a shape transformation to a shorter wider rod with a smaller aspect ratio at a temperature significantly below the bulk melting transition. The new shape, that forms upon slow heating, is now covered almost entirely by more stable ⟨111⟩ facets, and the internal fcc crystal structure has rotated to align with these new facets. In contrast, a rod which is predominantly covered by ⟨111⟩ facets, remains stable up until melting. We belive that this phenomena on the nanoscale is related to the macroscopic behavior of these surfaces, where it is known that the ⟨100⟩ and ⟨110⟩ surfaces disorder and roughen, respectively, below the bulk melting temperature, while the ⟨111⟩ surface remains stable and ordered up to, and perhaps even beyond, melting. However, the excitations leading to shape transformations in the nanorods appear to involve a combination of both surface and bulk effects, which, unlike for macroscopic samples, cannot be easily separated.
For further details, see Nano Lett. 5, 2174 (2005) and J. Comput. Theor. Nanosci. 4, 282 (2007).
For macroscopically large samples, the crystal shape is generally determined by the growth process, rather than a true minimization of the free energy, due to the extremely long times needed for atoms to diffuse across the sample dimensions. For nanosized clusters, however, this is not necessarily so, and rod shaped gold nanoclusters have been observed to buckle, bulge, and transform to shapes with smaller aspect ratios, upon heating. The ability to form stable clusters of specified shape is crucial to the integration of such nanoclusters in device applications. We find that the stability of such gold nanorods of a few thousand atoms is crucially determined by the orientation of their surface facets. When the long axis of the rod has substantial surface area covered by either ⟨100⟩ or ⟨110⟩ oriented facets (as in the picture to the left), we find that the rod undergoes a shape transformation to a shorter wider rod with a smaller aspect ratio at a temperature significantly below the bulk melting transition. The new shape, that forms upon slow heating, is now covered almost entirely by more stable ⟨111⟩ facets, and the internal fcc crystal structure has rotated to align with these new facets. In contrast, a rod which is predominantly covered by ⟨111⟩ facets, remains stable up until melting. We belive that this phenomena on the nanoscale is related to the macroscopic behavior of these surfaces, where it is known that the ⟨100⟩ and ⟨110⟩ surfaces disorder and roughen, respectively, below the bulk melting temperature, while the ⟨111⟩ surface remains stable and ordered up to, and perhaps even beyond, melting. However, the excitations leading to shape transformations in the nanorods appear to involve a combination of both surface and bulk effects, which, unlike for macroscopic samples, cannot be easily separated.
For further details, see Nano Lett. 5, 2174 (2005) and J. Comput. Theor. Nanosci. 4, 282 (2007).
|